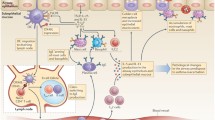
Abstract
Recognition of asthma as a heterogeneous disease revealed different potential molecular targets and urged the development of targeted, customized treatment modalities. Evidence was provided for different inflammatory subsets of asthma and more recently, further refined to T helper (Th)2-high and Th2-low subphenotypes with different responsiveness to standard and targeted pharmacotherapy. Given these differences in immunology and pathophysiology, proof of concept studies of novel treatment modalities for asthma should be performed in adequate, well-defined phenotypes. In this review, we describe both existing and novel biomarkers of Th2-inflammation in asthma that can be applied to classify asthma subphenotypes in clinical studies and for treatment monitoring.

Similar content being viewed by others
References
Global Initiative for Asthma (updated 2012), www.ginasthma.com.
Diamant Z, Boot JD, Mantzouranis E, et al. Biomarkers in asthma and allergic rhinitis. Pulm Pharmacol Ther. 2010;23(6):468–81.
Diamant Z, Boot JD, Virchow JC. Summing up 100 years of asthma. Respir Med. 2007;101(3):378–88.
Rosi E, Ronchi MC, Grazzini M, et al. Sputum analysis, bronchial hyperresponsiveness, and airway function in asthma: results of a factor analysis. J Allergy Clin Immunol. 1999;103:232–7.
Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24.
Moore WC, Meyers DA, Wenzel SE, et al. National Heart, Lung, and Blood Institute's severe asthma research program. Am J Respir Crit Care Med. 2010;181(4):315–23.
Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–13.
Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–63.
Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95.
Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130(4):829–42.
Jia G, Erickson RW, Choy DF, et al. Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma (BOBCAT) study group. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130(3):647–65.
Ruddy M, Zuiker R, Morelli N, et al. Detection of increased levels of Th2-derived cytokines in ultracentrifuged sputum following allergen challenge and their responsiveness to fluticasone. Am J Respir Crit Care Med. 2010:A4043.
Van der Pouw Kraan TC, Van der Zee JS, Boeije LC, et al. The role of IL-13 in IgE synthesis by allergic asthma patients. Clin Exp Immunol. 1998;111(1):129–35.
Besnard AG, Togbe D, Guillou N, et al. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41(6):1675–86.
Ramirez-Icaza G, Mohammed KA, Nasreen N, et al. Th2 cytokines IL-4 and IL-13 downregulate paxillin expression in bronchial airway epithelial cells. J Clin Immunol. 2004;24(4):426–34.
Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem. 2011;286(3):1786–94.
Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–88.
Chibana K, Trudeau JB, Mustovich AT, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38(6):936–46.
Venkayya R, Lam M, Willkom M, et al. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol. 2002;26(2):202–8.
Horn BR, Robin ED, Theodore J, Van-Kessel TA. Total eosinophil counts in the management of bronchial asthma. N Engl J Med. 1975;292:1152–5.
Ulrik CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995;108:10–5.
Krause JR, Boggs DR. Search for eosinopenia in hospitalized patients with normal blood leukocyte concentration. Am J Hematol. 1987;24(1):55–63.
Winkel P, Statland BE, Saunders AM, et al. Within-day physiologic variation of leukocyte types in healthy subjects as assayed by two automated leukocyte differential analyzers. Am J Clin Pathol. 1981;75(5):693–700.
Massanari M, Holgate ST, Busse WW, et al. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir Med. 2010;104(2):188–96.
Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol. 2003;131:46–52.
Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84.
Büttner C, Lun A, Splettstoesser T, et al. Monoclonal anti-interleukin-5 treatment suppresses eosinophil but not T-cell functions. Eur Respir J. 2003;21(5):799–803.
Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7.
Kerstjens HA, Schouten JP, Brand PL, et al. Importance of total serum IgE for improvement in airways hyperresponsiveness with inhaled corticosteroids in asthma and chronic obstructive pulmonary disease. The Dutch CNSLD Study Group. Am J Respir Crit Care Med. 1995;151(2 PT 1):360–8.
Ahmad Al Obaidi AH, Mohamed Al Samarai AG, Yahya Al Samarai AK, et al. The predictive value of IgE as biomarker in asthma. J Asthma. 2008;45:654–63.
Peona V, De Amici M, Quaglini S, et al. Serum eosinophilic cationic protein: is there a role in respiratory disorders? J Asthma. 2010;47:131–4.
Vatrella A, Ponticiello A, Parrella R. Serum eosinophil cationic protein (ECP) as a marker of disease activity and treatment efficacy in seasonal asthma. Allergy. 1996;51:547–55.
Nielsen LP, Peterson CG, Dahl R. Serum eosinophil granule proteins predict asthma risk in allergic rhinitis. Allergy. 2009;64(5):733–7.
Stelmach I, Jerzynska J, Kuna P. Markers of allergic inflammation in peripheral blood of children with asthma after treatment with inhaled triamcinolone acetonide. Ann Allergy Asthma Immunol. 2001;87(4):319–26.
Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, et al. Airway inflammation and eotaxin in exhaled breath condensate of patients with severe persistent allergic asthma during omalizumab therapy. Adv Med Sci. 2011;56(2):318–22.
Conroy DM, Jopling LA, Lloyd CM, et al. CCR4 blockade does not inhibit allergic airways inflammation. J Leukoc Biol. 2003;74(4):558–63.
Jahnz-Róyk K, Plusa T, Mierzejewska J. Eotaxin in serum of patients with asthma or chronic obstructive pulmonary disease: relationship with eosinophil cationic protein and lung function. Mediat Inflamm. 2000;9(3–4):175–9.
Pukelsheim K, Stoeger T, Kutschke D, et al. Cytokine profiles in asthma families depend on age and phenotype. PLoS One. 2010;5(12):e14299. 13.
Hoffmann HJ, Nielsen LP, Harving H, et al. Asthmatics able to step down from inhaled corticosteroid treatment without loss of asthma control have low serum eotaxin/CCL11. Clin Respir J. 2008;2(3):149–57.
Hijnen D, De Bruin-Weller M, Oosting B, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell- attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113(2):334–40.
Sugawara N, Yamashita T, Ote Y, et al. TARC in allergic disease. Allergy. 2002;57:180–1.
Sekiya T, Yamada H, Yamaguchi M, et al. Increased levels of a TH2-type CC chemokine thymus and activation regulated chemokine (TARC) in serum and induced sputum of asthmatics. Allergy. 2002;57:173–7.
Leung TF, Wong CK, Chan IH, et al. Plasma concentration of thymus and activation-regulated chemokine is elevated in childhood asthma. J Allergy Clin Immunol. 2002;110:404–9.
Bochner BS, Hudson SA, Xiao HQ, et al. Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J Allergy Clin Immunol. 2003;112(5):930–4.
ten Hacken NH, Oosterhoff Y, Kauffman HF, et al. Elevated serum interferon-gamma in atopic asthma correlates with increased airways responsiveness and circadian peak expiratory flow variation. Eur Respir J. 1998;11(2):312–6.
Koopmans JG, Lutter R, Jansen HM, van der Zee JS. Adding salmeterol to an inhaled corticosteroid reduces allergen-induced serum IL-5 and peripheral blood eosinophils. J Allergy Clin Immunol. 2005;116(5):1007–113.
Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; (ahead of print).
Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98.
Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma. Am J Respir Crit Care Med. 2013;187(8):804–11.
Venge P. Monitoring the allergic inflammation. Allergy. 2004;59(1):26–32.
Wolthers OD, Heuck C. Circadian variations in serum eosinophil cationic protein, and serum and urine eosinophil protein X. Pediatr Allergy Immunol. 2003;14:130–3.
Kristjansson S, Strannegård IL, Strannegård Ö, et al. Urinary eosinophil protein X in children with atopic asthma: a useful marker of antiinflammatory treatment. J Allergy Clin Immunol. 1996;97:1179–87.
Oymar K. High levels of urinary eosinophil protein X in young asthmatic children predict persistent atopic asthma. Pediatr Allergy Immunol. 2001;12:312–7.
Diamant Z, Sampson AP. Anti-inflammatory mechanisms of leukotriene modulators. Editorial. Clin Exp Allergy. 1999;29:1449–53.
Daffern PJ, Muilenburg D, Hugli TE, et al. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol. 1999;104:559–64.
Diamant Z, Timmers MC, van der Veen H, et al. The effect of MK-0591, a novel 5-lipoxygenase activating protein inhibitor, on leukotriene biosynthesis and allergen-induced airway responses in asthmatic subjects in vivo. J Allergy Clin Immunol. 1995;95:42–51.
Liu MC, Dube LM, Lancaster J. Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: a 6-month randomized multicenter trial. Zileuton Study Group. J Allergy Clin Immunol. 1996;98(5 Pt 1):859–71.
Cai C, Yang J, Hu S, et al. Relationship between urinary cysteinyl leukotriene E4 levels and clinical response to antileukotriene treatment in patients with asthma. Lung. 2007;185:105–12.
Sousa AR, Lams BE, Pfister R, et al. Expression of interleukin-5 and granulocyte-macrophage colony-stimulating factor in aspirin-sensitive and non-aspirin-sensitive asthmatic airways. Am J Respir Crit Care Med. 1997;156(5):1384–9.
Braunstahl GJ, Kleinjan A, Overbeek SE, et al. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000;161(6):2051–7.
Berry M, Hargadon B, Morgan A, et al. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur Respir J. 2005;25(6):986–91.
Overbeek SE, O'Sullivan S, Leman K, et al. Effect of montelukast compared with inhaled fluticasone on airway inflammation. Clin Exp Allergy. 2004;34(9):1388–94.
Van Rensen EL, Straathof KC, Veselic-Charvat MA, et al. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax. 1999;54(5):403–8.
Robinson DS, Assoufi B, Durham SR, et al. Eosinophil cationic protein (ECP) and eosinophil protein X (EPX) concentrations in serum and bronchial lavage fluid in asthma. Clin Exp Allergy. 1995;25(11):1118–27.
Vignola AM, Chanez P, Campbell AM, et al. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998;157(2):403–9.
de Blay F, Krieger P, Spirlet F, et al. Repeated inhalation of low doses of cat allergen that do not induce clinical symptoms increases bronchial hyperresponsiveness and eosinophil cationic protein levels. Int Arch Allergy Immunol. 1999;120(2):158–65.
Peebles Jr RS, Hamilton RG, Lichtenstein LM, et al. Antigen-specific IgE and IgA antibodies in bronchoalveolar lavage fluid are associated with stronger antigen-induced late phase reactions. Clin Exp Allergy. 2001;31(2):239–48.
Woodman L, Sutcliffe A, Kaur D, et al. Chemokine concentrations and mast cell chemotactic activity in BAL fluid in patients with eosinophilic bronchitis and asthma, and in normal control subjects. Chest. 2006;130(2):37137–8.
Feltis BN, Reid DW, Ward C, et al. BAL eotaxin and IL-5 in asthma, and the effects of inhaled corticosteroid and beta2 agonist. Respirology. 2004;9(4):507–13.
Becky Kelly EA, Busse WW, Jarjour NN. A comparison of the airway response to segmental antigen bronchoprovocation in atopic asthma and allergic rhinitis. J Allergy Clin Immunol. 2003;111(1):79–86.
Walker C, Bauer W, Braun RK, et al. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994;150(4):1038–48.
Batra V, Musani AI, Hastie AT, et al. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy. 2004;34(3):437–44.
Ali FR, Kay AB, Larché M. Airway hyperresponsiveness and bronchial mucosal inflammation in T cell peptide-induced asthmatic reactions in atopic subjects. Thorax. 2007;62(9):750–7.
Fajt ML, Gelhaus SL, Freeman B, et al. Prostaglandin D2 pathway upregulation: Relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;31(6):1504–12.
Wenzel SE, Trudeau JB, Kaminsky DA, et al. Effect of 5-lipoxygenase inhibition on bronchoconstriction and airway inflammation in nocturnal asthma. Am J Respir Crit Care Med. 1995;152(3):897–905.
Cowburn AS, Sladek K, Soja J, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101(4):834–46.
Szczeklik A, Sladek K, Dworski R, et al. Bronchial aspirin challenge causes specific eicosanoid response in aspirin-sensitive asthmatics. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1608–14.
Oosterhoff Y, Overbeek SE, Douma R, et al. Lower leukotriene C(4) levels in bronchoalveolar lavage fluid of asthmatic subjects after 2.5 years of inhaled corticosteroid therapy. Mediators Inflamm. 1995;4(6):426–30.
Bakakos P, Schleich F, Alchanatis M, et al. Induced sputum in asthma: from bench to bedside. Curr Med Chem. 2011;18(10):1415–22.
Sohn SW, Lee HS, Park HW, et al. Evaluation of cytokine mRNA in induced sputum from patients with allergic rhinitis: relationship to airway hyperresponsiveness. Allergy. 2008;63(3):268–73.
Gauvreau GM, Watson RM, Rerecich TJ, et al. Repeatability of allergen-induced airway inflammation. J Allergy Clin Immunol. 1999;104(1):66–71.
Clarke GW, Diamant Z, Greenaway SD, et al. Launching the BIOSPIT initiative: harmonizing sputum outcomes in multicenter trials. Pulm Pharmacol Ther. 2013;26(3):400–1.
Al Obaidi AH, Al Samarai AG, Al-Janabi J, et al. The predictive value of eosinophil cationic protein and lactate dehydrogenase in asthma: a comparative study of serum versus sputum. World Allergy Organ J. 2009;2(7):144–9.
Xu J, Jiang F, Nayeri F, Zetterström O. Apoptotic eosinophils in sputum from asthmatic patients correlate negatively with levels of IL-5 and eotaxin. Respir Med. 2007;101(7):1447–54.
Fujimoto K, Kubo K, Matsuzawa Y, et al. Eosinophil cationic levels in induced sputum correlate with the severity of bronchial asthma. Chest. 1997;112:1241–7.
Hanxiang N, Jiong Y, Yanwei C, et al. Persistent airway inflammation and bronchial hyperresponsiveness in patients with totally controlled asthma. Int J Clin Pract. 2008;62(4):599–605.
Broekema M, Volbeda F, Timens W, et al. Airway eosinophilia in remission and progression of asthma: accumulation with a fast decline of FEV(1). Respir Med. 2010;104(9):1254–62.
Schulze J, Voss S, Zissler U, et al. Airway responses and inflammation in subjects with asthma after four days of repeated high-single-dose allergen challenge. Respir Res. 2012;13:78.
Lee JH, Park KH, Park JW, et al. YKL-40 in induced sputum after allergen bronchial provocation in atopic asthma. J Investig Allergol Clin Immunol. 2012;22(7):501–7.
Lazarus SC, Chinchilli VM, Rollings NJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175(8):783–90.
Strauch E, Moske O, Thoma S, et al. A randomized controlled trial on the effect of montelukast on sputum eosinophil cationic protein in children with corticosteroid-dependent asthma. Pediatr Res. 2003;54(2):198–203.
Gauvreau GM, Boulet LP, Schmid-Wirlitsch C, et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir Res. 2011;12:140.
Dente FL, Carnevali S, Bartoli ML, et al. Profiles of proinflammatory cytokines in sputum from different groups of severe asthmatic patients. Ann Allergy Asthma Immunol. 2006;97:312–20.
Park SW, Jangm HK, An MH, et al. Interleukin-13 and interleukin-5 in induced sputum of eosinophilic bronchitis: comparison with asthma. Chest. 2005;128(4):1921–7.
Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121(3):560–70.
Komai-Koma M, McKay A, Thomson L, et al. Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clin Exp Allergy. 2001;31(9):1441–8.
Aggarwal S, Moodley YP, Thompson PJ, Misso NL. Prostaglandin E2 and cysteinyl leukotriene concentrations in sputum: association with asthma severity and eosinophilic inflammation. Clin Exp Allergy. 2010;40(1):85–93.
Tufvesson E, van Weele LJ, Ekedahl H, Bjermer L. Levels of cysteinyl-leukotrienes in exhaled breath condensate are not due to saliva contamination. Clin Respir J. 2010;4(2):83–8.
Tufvesson E, Aronsson D, Bjermer L. Cysteinyl-leukotriene levels in sputum differentiate asthma from rhinitis patients with or without bronchial hyperresponsiveness. Clin Exp Allergy. 2007;37(7):1067–73.
Hallstrand TS, Henderson Jr WR. Role of leukotrienes in exercise-induced bronchoconstriction. Curr Allergy Asthma Rep. 2009;9(1):18–25.
Gaber F, Daham K, Higashi A, et al. Increased levels of cysteinyl-leukotrienes in saliva, induced sputum, urine and blood from patients with aspirin-intolerant asthma. Thorax. 2008;63(12):1076–82.
Pavord I, Woodcock A, Parker D, et al. Salmeterol plus fluticasone propionate versus fluticasone propionate plus montelukast: a randomised controlled trial investigating the effects on airway inflammation in asthma. Respir Res. 2007;8:67.
Reid DW, Misso NL, Aggarwal S, et al. Tolerance and rebound with zafirlukast in patients with persistent asthma. J Negat Results Biomed. 2008;7:3.
Ricciardolo FL, Di Stefano A, Silvestri M, et al. Exhaled nitric oxide is related to bronchial eosinophilia and airway hyperresponsiveness to bradykinin in allergen-induced asthma exacerbation. Int J Immunopathol Pharmacol. 2012;25(1):175–82.
Dweik RA, Sorkness RL, Wenzel S, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181(10):1033–41.
Nair P, Kjarsgaard M, Armstrong S, et al. Nitric oxide in exhaled breath is poorly correlated to sputum eosinophils in patients with prednisone-dependent asthma. J Allergy Clin Immunol. 2010;126(2):404–6.
Redington AE, Meng QH, Springall DR, et al. Increased expression of inducible nitric oxide synthase and cyclo-oxygenase-2 in the airway epithelium of asthmatic subjects and regulation by corticosteroid treatment. Thorax. 2001;56(5):351–7.
Jiang J, Malavia N, Suresh V, George SC. Nitric oxide gas phase release in human small airway epithelial cells. Respir Res. 2009;10:3.
Suresh V, Mih JD, George SC. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2007;37(1):97–104.
Liang Y, Yeligar SM, Brown LA. Exhaled breath condensate: a promising source for biomarkers of lung disease. Sci World J. 2012;2012:217518.
Kazani S, Planaguma A, Ono E, et al. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J Allergy Clin Immunol. 2013, Apr 19. (in press).
Rathnayake N, Akerman S, Klinge B, et al. Salivary biomarkers for detection of systemic diseases. PLoS One. 2013;8(4):e61356.
Blicharz TM, Siqueira WL, Helmerhorst EJ, et al. Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva. Anal Chem. 2009;81(6):2106–14.
Compliance with Ethics Guidelines
Conflict of Interest
Zuzana Diamant has served on an advisory board (Aerocrine) and served as a consultant for various companies (Hall Allergy Hexal, Mundipharma, Urogenix, Profess, QPS Netherlands).
Leif Bjermer has served on advisory boards and received payment for giving lectures.
Ellen Tufvesson declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors.
Human Studies
With regard to the author’s research cited in this paper, all institutional and national guidelines for the care and use of laboratory animals were followed. In addition, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diamant, Z., Tufvesson, E. & Bjermer, L. Which Biomarkers Are Effective for Identifying Th2-Driven Inflammation in Asthma?. Curr Allergy Asthma Rep 13, 477–486 (2013). https://doi.org/10.1007/s11882-013-0376-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-013-0376-6