Abstract
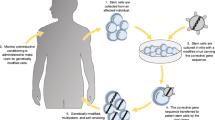
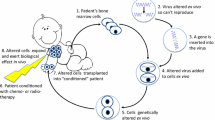
Since the early 1990s, primary immunodeficiency (ID) disorders have played a major role in the development of human gene therapy. Adenosine deaminase (ADA) deficiency was the first disease to be treated with a gene therapy approach in humans, and was also the first condition for which therapeutic gene transfer into the hematopoietic stem cell has been attempted in the clinical arena. A series of encouraging results obtained in chronic granulomatous disease (CGD) pateints have followed these pioneer experiments and preceded the very recent and exciting reports of successful genetic correction procedures performed in patients affected with the X-linked form of severe combined immunodeficiency (XSCID). The technical progress made in the field of gene transfer in recent years is mostly responsible for these clinical advances, and will be critical for future development of gene therapy approaches for other forms of IDs.
Similar content being viewed by others
References and Recommended Reading
Ziegler BL, Valtieri M, Porada GA, et al.: KDR receptor: a key marker defining hematopoietic stem cells. Science 1999, 285:1553–1558.
Dao MA, Nolta JA: CD34: to select or not to select? That is the question. Leukemia 2000, 14:773–776.
Blaese RM, Culver KW, Miller AD, et al.: T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science 1995, 270:475–480.
Bordignon C, Notarangelo LD, Nobili N, et al.: Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science 1995, 270:470–475.
Onodera M, Ariga T, Kawamura N, et al.: Successful peripheral T lymphocyte-directed gene transfer for a patient with severe combined immune deficiency due to adenosine deaminase deficiency. Blood 1998, 91:30–36. The results of this trial confirm previous clinical findings by Blaese et al. [3] and indicate T-cell gene therapy as a viable option for the treatment of ADA deficiency.
Buckley RH, Schiff SE, Schiff RI, et al.: Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med 1999, 340:508–516.
Hershfield MS: Adenosine deaminase deficiency: clinical expression, molecular basis, and therapy. Semin Hematol 1998, 35:291–298.
Kohn DB, Hershfield MS, Carbonaro D, et al.: T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates. Nat Med 1998, 4:775–780. This article describes the results of 4.5 years of follow-up after the treatment of three ADA-deficient newborns with gene-corrected umbilical cord CD34+ cells, and it provides evidence of survival advantage of gene-corrected T lymphocytes over unmodified cells. In addition, it reports on the unsuccessful attempt to withdraw PEGADA replacement therapy from one of the patients, indicating the need for higher levels of expression of the therapeutic ADA gene.
Hoogerbrugge PM, van Beusechem VW, Fischer A, et al.: Bone marrow gene transfer in three patients with adenosine deaminase deficiency. Gene Ther 1996, 3:179–183.
Sekhsaria S, Gallin JI, Linton GF, et al.: Peripheral blood progenitors as a target for genetic correction of p47phoxdeficient chronic granulomatous disease. Proc Natl Acad Sci U S A 1993, 90:7446–7450.
Li F, Linton GF, Sekhsaria S, et al.: CD34+ peripheral blood progenitors as a target for genetic correction of the two flavocytochrome b558 defective forms of chronic granulomatous disease. Blood 1994, 84:53–58.
Mardiney MR, Jackson SH, Spratt SK, et al.: Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood 1997, 89:2268–2275.
Bjorgvinsdottir H, Ding C, Pech N, et al.: Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against Aspergillus fumigatus in murine X-linked chronic granulomatous disease. Blood 1997, 89:41–48.
Malech HL, Maples PB, Whiting-Theobald N, et al.: Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc Natl Acad Sci U S A 1997, 94:12133–12138.
Malech HL, Horwitz ME, Linton GF, et al.: Extended production of oxidase normal neutrophils in X-linked chronic granulomatous disease (CGD) following gene therapy with gp91(phox) transduced CD34+ cells [abstract]. Blood 1998, 92:690A. This abstract describes the preliminary observations made in a group of three patients treated by Malech et al. [14] using improved technical procedures compared with the procedures used in the previous clinical trial.
Fischer A, Lisowska-Grospierre B, Anderson DC, et al.: Leukocyte adhesion deficiency: molecular basis and functional consequences. Immunodefic Rev 1988, 1:39–54.
Yorifuji T, Wilson RW, Beaudet AL: Retroviral mediated expression of CD18 in normal and deficient human bone marrow progenitor cells. Hum Mol Genet 1993, 2:1443–1448.
Bauer TR, Schwartz BR, Conrad Liles W, et al.: Retroviralmediated gene transfer of the leukocyte integrin CD18 into peripheral blood CD34+ cells derived from a patient with leukocyte adhesion deficiency type 1. Blood 1998, 91:1520–1526.
Bauer TR, Hickstein DD: Gene therapy for leukocyte adhesion deficiency. Curr Opin Mol Ther 2000, 2:383–388. This review article contains a detailed description of the results obtained in two LAD-1 patients treated with corrective gene transfer into G-CSF-mobilized peripheral blood CD34+ cells.
Buckley RH: Advances in immunology: primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med 2000, 343:1313–1324.
Sugamura K, Asao H, Kondo M, et al.: The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol 1996, 14:179–205.
Haddad E, Landais P, Friedrich W, et al.: Long-term immune reconstitution and outcome after HLA-nonidentical T-celldepleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood 1998, 91:3646–3653.
Candotti F, Johnston JA, Puck JM, et al.: Retroviral-mediated gene correction for X-linked severe combined immunodeficiency. Blood 1996, 87:3097–3102.
Taylor N, Uribe L, Smith S, et al.: Correction of interleukin-2 receptor function in X-SCID lymphoblastoid cells by retrovirally mediated transfer of the gamma-c gene. Blood 1996, 87:3103–3107.
Hacein-Bey S, Cavazzana-Calvo M, Le Deist F, et al.: Gamma-c gene transfer into SCID X1 patients’ B-cell lines restores normal high-affinity interleukin-2 receptor expression and function. Blood 1996, 87:3108–3116.
Cavazzana-Calvo M, Hacein-Bay S, de Saint Basile G, et al.: Role of interleukin-2 (IL-2), IL-7, and IL-15 in natural killer cell differentiation from cord blood hematopoietic progenitor cells and from gc transduced severe combined immunodeficiency X1 bone marrow cells. Blood 1996, 88:3901–3909.
Hacein-Bey S, Basile GD, Lemerle J, et al.: gammac gene transfer in the presence of stem cell factor, FLT-3L, interleukin-7 (IL-7), IL-1, and IL-15 cytokines restores T-cell differentiation from gammac(-) X-linked severe combined immunodeficiency hematopoietic progenitor cells in murine fetal thymic organ cultures. Blood 1998, 92:4090–4097.
Lo M, Bloom ML, Imada K, et al.: Restoration of lymphoid populations in a murine model of X-linked severe combined immunodeficiency by a gene-therapy approach. Blood 1999, 94:3027–3036. This article demonstrated the safety and efficacy of gc gene transfer into the hematopoietic stem cell in murine models of X-SCID and has supported the development of human trials for gene therapy of X-SCID.
Otsu M, Anderson SM, Bodine DM, et al.: Lymphoid development and function in X-linked severe combined immunodeficiency mice after stem cell gene therapy. Mol Ther 2000, 1:145–153. This article demonstrated the safety and efficacy of gc gene transfer into the hematopoietic stem cell in murine models of X-SCID and has supported the development of human trials for gene therapy of X-SCID.
Soudais C, Shiho T, Sharara LI, et al.: Stable and functional lymphoid reconstitution of common cytokine receptor gamma chain deficient mice by retroviral-mediated gene transfer. Blood 2000, 95:3071–3077. This article demonstrated the safety and efficacy of gc gene transfer into the hematopoietic stem cell in murine models of X-SCID and has supported the development of human trials for gene therapy of X-SCID.
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al.: Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000, 288:669–672. This report describes the successful immunologic reconstitution observed in two patients with X-SCID who were treated with autologous bone marrow-derived CD34+ cells transduced with a retroviral vector expressing the human gc. Data relative to a 9-month follow-up are presented.
Markert ML: Purine nucleoside phosphorylase deficiency. Immunodefic Rev 1991, 3:45–81.
Nelson DM, Butters KA, Markert ML, et al.: Correction of proliferative responses in purine nucleoside phosphorylase (PNP)-deficient T lymphocytes by retroviral-mediated PNP gene transfer and expression. J Immunol 1995, 154:3006–3014.
Macchi P, Villa A, Giliani S, et al.: Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 1995, 377:65–68.
Russell SM, Tayebi N, Nakajima H, et al.: Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 1995, 270:797–800.
Candotti F, Oakes SA, Johnston JA, et al.: Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood 1997, 90:3996–4003.
Candotti F, Oakes S, Johnston JA, et al.: In vitro correction of JAK3-deficient severe combined immunodeficiency by retroviral- mediated gene transduction. J Exp Med 1996, 183:2687–2692.
Bunting KD, Sangster MY, Ihle JN, et al.: Restoration of lymphocyte function in Janus kinase 3-deficient mice by retroviral-mediated gene transfer. Nat Med 1998, 4:58–64. An in vivo model of retroviral-mediated gene therapy for JAK3 deficiency demonstrated the safety and efficacy of this procedure in vivo and supported the development of similar strategies for human trials.
Notarangelo LD, Hayward AR: X-linked immunodeficiency with hyper-IgM (XHIM). Clin Exp Immunol 2000, 120:399–405.
Brown MP, Topham DJ, Sangster MY, et al.: Thymic lymphoproliferative disease after successful correction of CD40 ligand deficiency by gene transfer in mice. Nat Med 1998, 4:1253–1260. A strong cautionary note comes from the experiments described in this article, which showed the onset of malignant lymphoproliferation in mice subjected to a gene therapy approach leading to deregulated expression of CD40 ligand in lymphocyte progenitors.
Sabatino DE, Do BQ, Pyle LC, et al.: Amphotropic or gibbon ape leukemia virus retrovirus binding and transduction correlates with the level of receptor mRNA in human hematopoietic cell lines. Blood Cells Mol Dis 1997, 23:422–433.
Eckert HG, Stockschlader M, Just U, et al.: High-dose multidrug resistance in primary human hematopoietic progenitor cells transduced with optimized retroviral vectors. Blood 1996, 88:3407–3415.
Onodera M, Nelson DM, Yachie A, et al.: Development of improved adenosine deaminase retroviral vectors. J Virol 1998, 72:1769–1774.
Halene S, Wang L, Cooper RM, et al.: Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector. Blood 1999, 94:3349–3357.
Piacibello W, Sanavio F, Severino A, et al.: Engraftment in nonobese diabetic severe combined immunodeficient mice of human CD34(+) cord blood cells after ex vivo expansion: evidence for the amplification and self-renewal of repopulating stem cells. Blood 1999, 93:3736–3749.
Kiem HP, Andrews RG, Morris J, et al.: Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood 1998, 92:1878–1886.
Tisdale JF, Hanazono Y, Sellers SE, et al.: Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood 1998, 92:1131–1141.
Gatti RA, Meuwissen HJ, Allen HD, et al.: Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 1968, 2:1366–1369.
Hershfield MS, Buckley RH, Greenberg ML, et al.: Treatment of adenosine deaminase deficiency with polyethylene glycolmodified adenosine deaminase. N Engl J Med 1987, 316:589–596.
Uchida N, Sutton RE, Friera AM, et al.: HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci U S A 1998, 95:11939–11944.
Candotti F, Facchetti F, Blanzuoli L, et al.: Retrovirus-mediated WASP gene transfer corrects defective actin polymerization in B cell lines from Wiskott-Aldrich syndrome patients carrying ’null’ mutations. Gene Ther 1999, 6:1170–1174.
Huang MM, Tsuboi S, Wong A, et al.: Expression of human Wiskott-Aldrich syndrome protein in patients’ cells leads to partial correction of a phenotypic abnormality of cell surface glycoproteins. Gene Ther 2000, 7(4):314–320.
Klein C, Nguyen D, Liu CH, et al.: Restoration of wasp-deficient T-cell signaling defects in mice upon transplantation of retrovirally transduced hematopoietic stem cells. Blood 2000, 96:2535.
Islam TC, Branden LJ, Kohn DB, et al.: BTK mediated apoptosis, a possible mechanism for failure to generate high tilter retroviral producer clones. J Gene Med 2000, 2(3):204–209.
Yu PW, Tabuchi RS, Kato RM: Correction of X-linked immunodeficiency by retroviral mediated transfer of Bruton’s tyrosine kinase. Blood 2000, 96:896.
Taylor N, Bacon KB, Smith S, et al.: Reconstitution of T cell receptor signaling in ZAP-70-deficient cells by retroviral transduction of the ZAP-70 gene. J Exp Med 1996, 184(5):2031–2036.
Steinberg M, Swainson L, Schwarz K, et al.: Retrovirusmediated transduction of primary ZAP-70-deficient human T cells results in the selective growth advantage of genecorrected cells: implications for gene therapy. Gene Ther 2000, 7:1392–1400.
Bradley MB, Fernandez JM, Ungers G, et al.: Correction of defective expression in MHC class II deficiency (bare lymphocyte syndrome) cells by retroviral transduction of CIITA. J Immunol 1997, 159(3):1086–1095.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Candotti, F. Gene therapy for immunodeficiency. Curr Allergy Asthma Rep 1, 407–415 (2001). https://doi.org/10.1007/s11882-001-0025-3
Issue Date:
DOI: https://doi.org/10.1007/s11882-001-0025-3