Opinion statement
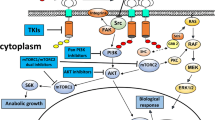
Extensive treatment-related morbidities and stagnant survival rates over the past few decades for patients with squamous cell cancer of the head and neck (SCCHN) emphasize the need for novel diagnostics and therapeutics based on the molecular characteristics of the tumor. The development of an early detection test remains largely preliminary. Much attention has recently been given to saliva-based early detection assays that use accepted tumor markers such as p53 and DNA methylation. Most of these studies have focused on feasibility as opposed to prospective clinical trials. To date, early detection saliva assays have failed to yield a high enough sensitivity and specificity for broad population-based screening. The use of saliva as a noninvasive, inexpensive, and accessible diagnostic substrate remains desirable. Unlike SCCHN diagnostics, molecular-targeted therapies for SCCHN will soon be a reality, with many more compounds in the pipeline. The most promising of these drugs target the epidermal growth factor receptor (EGFR), which is known to be overexpressed in squamous cell carcinomas. Cetuximab, a monoclonal EGFR antibody, has shown efficacy in combination with radiotherapy in advanced SCCHN in a recent phase III trial and is currently being petitioned for US Food and Drug Administration approval. Likewise, erlotinib, an EGFR tyrosine kinase inhibitor, has shown favorable results in phase II trials as monotherapy and in combination with chemotherapy. Gefitinib, another EGFR tyrosine kinase inhibitor, has shown efficacy as monotherapy, in combination with chemotherapy, and with chemoradiotherapy. At least two phase III trials of gefitinib in patients with advanced SCCHN are ongoing. Such low-toxicity, tumor-specific targeting strategies will soon be available for patients with head and neck cancer. The challenge is to establish assays to determine which patients are most likely to benefit from these agents.
Similar content being viewed by others
References and Recommended Reading
Miller C, Koeffler HP: p53 mutations in human cancer. Leukemia 1993, 7(Suppl 2):S18-S21.
Liu T, Wahlberg S, Burek E, et al.: Microsatellite instability as a predictor of a mutation in a DNA mismatch repair gene in familial colorectal cancer. Genes Chromosomes Cancer 2000, 27:17–25.
Groden J, Thliveris S, Samowitz W, et al.: Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66:589–600.
Sidransky D: Nucleic acid-based methods for the detection of cancer. Science 1997, 278:1054–1059.
Malamud D: Oral diagnostic testing for detecting human immunodeficiency virus-1 antibodies: a technology whose time has come. Am J Med 1997, 102:9–14.
Guven Y, Satman I, Dinccag N, et al.: Salivary peroxidase activity in whole saliva of patients with insulindependent (type 1) diabetes mellitus. J Clin Periodontol 1996, 23:879–881.
Streckfus C, Bigler L, Dellinger T, et al.: The presence of soluble c-erbB-2 in saliva and serum among women with breast carcinoma: a preliminary study. Clin Cancer Res 2000, 6:2363–2370.
Califano JA, Ahrendt S, Meininger G, et al.: Detection of telomerase activity in oral rinses from head and neck squamous cell cancer patients. Cancer Res 1996, 56:5720–5722.
Fliss MS, Usadel H, Caballero OL, et al.: Facile detection of mitochondrial DNA mutations in tumors and bodily fluid. Science 2000, 287:2017–2019.
Li Y, St John MA, Zhou X, et al.: Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res 2004, 10:8442–8450. This paper discusses the results of the only mRNA -based saliva SCCHN diagnostic assay to date. However, the sensitivity and specificity reported are not stringent enough for a populationbased screening diagnostic.
Bugart L, Zheng J, Shu Q, et al.: Somatic mitochondrial mutation in gastric cancer. Am J Pathol 1995, 147:1105–1111.
Habano W, Nakamura S, Sugai T: Microsatellite instability and mutation of mitochondrial and nuclear DNA in gastric carcinoma. Gastroenterology 2000, 118:835–841.
Jones JB, Song JJ, Hempen PM, et al.: Detection of mitochondrial DNA mutations in pancreatic cancer offers a “mass≓ -ive advantage over detection of nuclear DNA mutations. Cancer Res 2001, 61:1299–1304.
Horton TM, Petros JA, Heddi A, et al.: Novel mitochondrial DNA deletion found in a renal cell carcinoma. Genes Chromosomes Cancer 1996, 15:95–101.
Bianchi MS, Bianchi NO, Bailliet G: Mitochondrial DNA mutations in normal and tumor tissues from breast cancer patients. Cytogenet Cell Genet 1995, 71:99–103.
Sanchez -Cespedes M, Parella P, Nomoto S, et al.: Identification of a mononucleotide repeat as a major target for mitochondrial DNA alterations in human tumors. Cancer Res 2002, 61:7015–7019.
Lee HC, Lu CY, Fahn HJ, et al.: Aging - and smoking-associated alteration in the relative content of mitochondrial DNA in human lung. FEBS Lett 1998, 441:282–288.
Coller HA, Khrapko K, Bodyak ND, et al.: High frequency of homoplasmic mitochondrial DNA mutations in human tumors can be explained without selection. Nat Genet 2001, 28:147–150.
Jiang WW, Masayesva B, Zahurak M, et al.: Increased mitochondrial DNA content in saliva associated with head and neck cancer. Clin Cancer Res 2005, 11:2486–2491. This study is the first large-scale saliva diagnostic test to use mtDNA. mtDNA levels were increased for SCCHN patients and smokers within the control group.
Jones PA, Laird PW: Cancer epigenetics comes of age. Nat Genet 1999, 21:163–167.
Baylin SB, Herman JG: DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet 2000, 16:168–174.
Van der Riet P, Nawroz H, Hruban RH, et al.: Frequent loss of chromosome 9p21-22 early in head and neck cancer progression. Cancer Res 1994, 54:1156–1158.
Somers KD, Merrick MA, Lopez ME, et al.: Frequent p53 mutations in head and neck cancer. Cancer Res 1992, 52:5997–6000.
Sanchez -Cespedes M, Esteller M, Wu L, et al.: Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res 2000, 60:892–895.
Hasegawa M, Nelson HH, Peters E, et al.: Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene 2002, 21:4231–4236.
Kim DH, Nelson HH, Wiencke JK, et al.: P16(INK4a) and histology-specific methylation of CpG islands by exposure to tobacco smoke in non-small cell lung cancer. Cancer Res 2001, 61:3419–3424.
Van Engeland M, Weijenberg MP, Roemen GM, et al.: Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res 2003, 63:3133–3137.
Rosas SL, Koch W, da Costa Carvalho MG, et al.: Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and deathassociated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res 2001, 61:939–942.
Bosch FX, Manos MM, Munoz N, et al.: Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) study group. J Natl Cancer Inst 1995, 87:796–802.
Gillison ML, Koch WM, Capone RB, et al.: Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000, 92:709–720.
Werness BA, Levine AJ, Howley PM: Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990, 248:76–79.
Boyer SN, Wazer DE, Band V: E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through ubiquitin-proteasome pathway. Cancer Res 1996, 56:4620–4624.
Herrero R, Castellsague X, Pawlita M, et al.: Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003, 95:1772–1783.
Smith EM, Ritchie JM, Summersgill KF, et al.: Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer 2004, 108:766–772.
Zhao M, Rosenbaum E, Carvalho AL, et al.: Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. Int J Cancer 2005, 117:605–610.
Mao L, Lee DJ, Tockman MS, et al.: Microsatellite alterations as clonal markers for the detection of human cancer. Proc Natl Acad Sci U S A 1994, 91:9871–9875.
El Naggar AK, Hurr K, Huff V, et al.: Microsatellite instability in preinvasive and invasive head and neck squamous carcinoma. Am J Pathol 1996, 148:2067–2072.
Spafford MF, Koch WM, Reed AL, et al.: Detection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by microsatellite analysis. Clin Cancer Res 2001, 7:607–612.
Dancey J, Sausville EA: Issues and progress with protein kinase inhibitors for the treatment of cancer. Nat Rev Drug Discov 2003, 2:296–313. This paper presents an excellent overview of EGFR-based receptor tyrosine kinase inhibitors.
Rubin Grandis J, Tweardy DJ: Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 1993, 53:3579–3584.
Goldstein NI, Prewett M, Zuklys K, et al.: Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1995, 1:1311–1318.
Baselga J: The EGFR as a target for anticancer therapy-focus on cetuximab. Eur J Cancer 2001, 37(Suppl 4):S16-S22.
Burtness BA, Li Y, Flood W, et al.: Phase III trial comparing cisplatin (C) + placebo (P) to C + antiepidermal growth factor antibody (EGF-R) C225 in patients (pts) with metastatic/recurrent head & neck cancer (HNC). Proc Am Soc Clin Oncol 2002, 21:901.
Bonner JA, Giralt J, Harari PM, et al.: Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head and neck: a phase III study of high dose radiation therapy with or without cetuximab. J Clin Oncol 2004, 22:5007. This study is the basis for the pending FDA approval of cetuximab for SCCHN. Toxicity was minimal, and a significant improvement in 2-and 3-year survival rates was reported.
Trigo J, Hitt R, Koralewski P, et al.: Cetuximab monotherapy is active in patients (pts) with platinumrefractory recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN): results of a phase II study. Proc Am Soc Clin Oncol 2004, 23:5502.
Fry DW, Bridges AJ, Denny WA, et al.: Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci U S A 1998, 95:12022–12027.
Moyer JD, Barbacci EG, Iwata KK, et al.: Induction of apoptosis and cell cycle arrest by CP-358.774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 1997, 57:4838–4848.
Shepherd FA, Rodrigues Pereira J, et al.: Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005, 353:123–132.
Moore MJ, Goldstein D, Hamm J, et al.: Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG]. J Clin Oncol 2005, 23:1.
Soulieres D, Senzer NN, Vokes EE, et al.: Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004, 22:77–85.
Kim ES, Kies M, Sabichi A, et al.: Phase II study of combination cisplatin, docetaxel and erlotinib in patients with metastatic/recurrent head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2005, 23:5546.
Giaccone G, Herbst RS, Manegold C, et al.: Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial-INTACT1. J Clin Oncol 2004, 22:777–784.
Herbst RS, Giaccone G, Schiller JH, et al.: Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial-INTACT2. J Clin Oncol 2004, 22:785–794.
Han SW, Gwang PG, Chung DH, et al.: Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int J Cancer 2005, 113:109–115.
Cohen EE, Rosen F, Stadler WM, et al.: Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 2003, 21:1980–1987.
Murphy B, Li Y, Cella D, et al.: Phase III study comparing cisplatin (c) and 5-flurouracil (F) versus cisplatin and paclitaxel (T) in metastatic/recurrent head and neck cancer (MHN C). Proc Am Soc Clin Oncol 2001, 20:894.
Belon J, Irigoyen I, Rodriguez Y, et al.: Preliminary results of a phase II study to evaluate gefitinib combined with docetaxel and cisplatin in patients with recurrent and/or metastatic squamous-cell carcinoma of the head and neck. J Clin Oncol 2005, 23:5563.
Haraf DJ, Rosen FR, Stenson K, et al.: Induction chemotherapy followed by concomitant TFHX chemoradiotherapy with reduced dose radiation in advanced head and neck cancer. Clin Cancer Res 2003, 9:5936–5943.
Cohen EE, Haraf DJ, Stenson KM, et al.: Integration of gefitinib (G) into a concurrent chemoradiation (CRT) regimen followed by G adjuvant therapy in patients with locally advanced head and neck cancer (HNC)-a phase II trial. J Clin Oncol 2005, 23:5506.
Brizel DM, Sibley GS, Prosnitz LR, et al.: Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997, 38:285–289.
Rischin D, Peters L, Fisher R, et al.: Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol 2005, 23:79–87. This paper details encouraging results for non-EGFR-based therapy and provides a rationale for an ongoing phase III trial. The addition of tirapazamine significantly increased 3-year failure-free survival and locoregional control rates.
Vokes EE, Cohen EE, Mauer AM, et al.: A phase I study of erlotinib and bevacizumab for recurrent or metastatic squamous cell carcinoma of the head and neck (HNC). J Clin Oncol 2005, 23:5504.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chai, R.L., Grandis, J.R. Advances in molecular diagnostics and therapeutics in head and neck cancer. Curr. Treat. Options in Oncol. 7, 3–11 (2006). https://doi.org/10.1007/s11864-006-0027-4
Issue Date:
DOI: https://doi.org/10.1007/s11864-006-0027-4