Abstract
Introduction
Osteogenesis imperfecta (OI) is a genetic disorder characterized by bone fragility and fractures. Patients with OI have clinical features that may range from mild symptoms to severe bone deformities and neonatal lethality. Numerous approaches for the classification of OI have been published. The Sillence classification is the most commonly used. In this study, we aimed at developing a more refined sub-classification by applying a proposed scoring system for the quantitative assessment of clinical severity in different types of OI.
Subjects and methods
This study included 43 patients with OI. Clinical examination and radiological studies were conducted for all patients. Cases were classified according to the Sillence classification into types I–IV. The proposed scoring system included five major criteria of high clinical value: number of fractures per year, motor milestones, long bone deformities, length/height standard deviation score (SDS), and bone mineral density (BMD). Each criterion was assigned a score from 1 to 4, and each patient was marked on a scale from 1 to 20 according to these five criteria.
Results
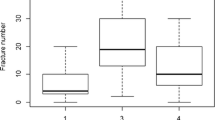
Applying the proposed clinical scoring system showed that all 11 patients with Sillence type I (100%) had a score between 6 and 10, denoting mild affection. The only patient with Sillence type II had a score of 19, denoting severe affection. In Sillence type III, 7 patients (31.8%) were moderately affected and 15 patients (68.2%) were severely affected. Almost all patients with Sillence type IV (88.9%) were moderately affected.
Conclusions
Applying the proposed scoring system can quantitatively reflect the degree of clinical severity in OI patients and can be used in complement with the Sillence classification and molecular studies.

Similar content being viewed by others
References
Martin E, Shapiro JR (2007) Osteogenesis imperfecta: epidemiology and pathophysiology. Curr Osteoporos Rep 5:91–97
Blumsohn A, McAllion SJ, Paterson CR (2001) Excess paternal age in apparently sporadic osteogenesis imperfecta. Am J Med Genet 100:280–286
Cabral WA, Marini JC (2004) High proportion of mutant osteoblasts is compatible with normal skeletal function in mosaic carriers of osteogenesis imperfecta. Am J Hum Genet 74:752–760
Marini JC, Cabral WA, Barnes AM (2010) Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res 339:59–70
Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC (2006) Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med 355:2757–2764
Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, Castagnola P, Rauch F, Glorieux FH, Vranka J, Bächinger HP, Pace JM, Schwarze U, Byers PH, Weis M, Fernandes RJ, Eyre DR, Yao Z, Boyce BF, Lee B (2006) CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 127:291–304
Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, Leikin S, Makareeva E, Kuznetsova NV, Rosenbaum KN, Tifft CJ, Bulas DI, Kozma C, Smith PA, Eyre DR, Marini JC (2007) Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet 39:359–365. Erratum: Nat Genet 40:927, 2008
Drögemüller C, Becker D, Brunner A, Haase B, Kircher P, Seeliger F, Fehr M, Baumann U, Lindblad-Toh K, Leeb T (2009) A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet 5:e1000579
van Dijk FS, Nesbitt IM, Zwikstra EH, Nikkels PGJ, Piersma SR, Fratantoni SA, Jimenez CR, Huizer M, Morsman AC, Cobben JM, van Roij MHH, Elting MW, Verbeke JI, Wijnaendts LC, Shaw NJ, Högler W, McKeown C, Sistermans EA, Dalton A, Meijers-Heijboer H, Pals G (2009) PPIB mutations cause severe osteogenesis imperfecta. Am J Hum Genet 85:521–527
Alanay Y, Avaygan H, Camacho N, Utine GE, Boduroglu K, Aktas D, Alikasifoglu M, Tuncbilek E, Orhan D, Bakar FT, Zabel B, Superti-Furga A, Bruckner-Tuderman L, Curry CJ, Pyott S, Byers PH, Eyre DR, Baldridge D, Lee B, Merrill AE, Davis EC, Cohn DH, Akarsu N, Krakow D (2010) Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 86:551–559
Christiansen HE, Schwarze U, Pyott SM, AlSwaid A, Al Balwi M, Alrasheed S, Pepin MG, Weis MA, Eyre DR, Byers PH (2010) Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet 86:389–398
Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, Bergmann C, Rohrbach M, Koerber F, Zimmermann K, de Vries P, Wirth B, Schoenau E, Wollnik B, Veltman JA, Hoischen A, Netzer C (2011) Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 88:362–371
Lapunzina P, Aglan M, Temtamy S, Caparrós-Martín JA, Valencia M, Letón R, Martínez-Glez V, Elhossini R, Amr K, Vilaboa N, Ruiz-Perez VL (2010) Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet 87:110–114
Martínez-Glez V, Valencia M, Caparrós-Martín JA, Aglan M, Temtamy S, Tenorio J, Pulido V, Lindert U, Rohrbach M, Eyre D, Giunta C, Lapunzina P, Ruiz-Perez VL (2012) Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat 33:343–350. doi:10.1002/humu.21647
Falvo KA, Root L, Bullough PG (1974) Osteogenesis imperfecta: clinical evaluation and management. J Bone Joint Surg Am 56:783–793
Bauze RJ, Smith R, Francis MJ (1975) A new look at osteogenesis imperfecta. a clinical, radiological and biochemical study of forty-two patients. J Bone Joint Surg Am 57:2–12
Sillence DO, Senn A, Danks DM (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16:101–116
Hanscom DA, Winter RB, Lutter L, Lonstein JE, Bloom BA, Bradford DS (1992) Osteogenesis imperfecta. Radiographic classification, natural history, and treatment of spinal deformities. J Bone Joint Surg Am 74:598–616
Plotkin H (2004) Syndromes with congenital brittle bones. BMC Pediatr 4:16
Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, LeMerrer M, Mortier G, Mundlos S, Nishimura G, Rimoin DL, Robertson S, Savarirayan R, Sillence D, Spranger J, Unger S, Zabel B, Superti-Furga A (2011) Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A 155A:943–968
Online Mendelian Inheritance in Man (OMIM). McKusick-Nathans Institute for Genetic Medicine, Johns Hopkins University (Baltimore, MD), and National Center for Biotechnology Information, National Library of Medicine (Bethesda, MD). Home page at: http://www.ncbi.nlm.nih.gov/omim
van Dijk FS, Pals G, Van Rijn RR, Nikkels PG, Cobben JM (2010) Classification of osteogenesis imperfecta revisited. Eur J Med Genet 53:1–5
Daly K, Wisbeach A, Sanpera I Jr, Fixsen JA (1996) The prognosis for walking in osteogenesis imperfecta. J Bone Joint Surg Br 78:477–480
Ghalli I, Salah N, Hussien F, Erfan M, El-Ruby M, Mazen I, Aglan MS, Hosny L, Zaki M, Ismail S, Elgammal M, Abd El-Dayem S et al. In: Sartorio A, Buckler JMH, Marazzi N (eds) Proceedings of the 1st National Congress for Egyptian Growth Curves, Cairo University, Cairo, Egypt, 11 December 2003. Published in Cresceve Nelmondo (2008). Ferring Company. Egyptian Growth Curves 2002 for Infants, Children and Adolescents
Chavassieux P, Seeman E, Delmas PD (2007) Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev 28:151–164
Rauch F, Lalic L, Roughley P, Glorieux FH (2010) Genotype–phenotype correlations in nonlethal osteogenesis imperfecta caused by mutations in the helical domain of collagen type I. Eur J Hum Genet 18:642–647
Ruck-Gibis J, Plotkin H, Hanley J, Wood-Dauphinee S (2001) Reliability of the gross motor function measure for children with osteogenesis imperfecta. Pediatr Phys Ther 13:10–17
Engelbert RH, Uiterwaal CS, Gulmans VA, Pruijs H, Helders PJ (2000) Osteogenesis imperfecta in childhood: prognosis for walking. J Pediatr 137:397–402
National Institutes of Health (NIH) Clinical Center (CC) (2007) Growth Hormone Therapy in Osteogenesis Imperfecta. National Institutes of Health Clinical Center, 9000 Rockville Pike, Bethesda, MD, prpl@mail.cc.nih.gov
Lund AM, Müller J, Skovby F (1999) Anthropometry of patients with osteogenesis imperfecta. Arch Dis Child 80:524–528
Rowe DW, Shapiro JR, Poirier M, Schlesinger S (1985) Diminished type I collagen synthesis and reduced alpha 1(I) collagen messenger RNA in cultured fibroblasts from patients with dominantly inherited (type I) osteogenesis imperfecta. J Clin Invest 76:604–611
Rauch F, Lalic L, Roughley P, Glorieux FH (2010) Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. J Bone Miner Res 25:1367–1374
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aglan, M.S., Hosny, L., El-Houssini, R. et al. A scoring system for the assessment of clinical severity in osteogenesis imperfecta. J Child Orthop 6, 29–35 (2012). https://doi.org/10.1007/s11832-012-0385-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11832-012-0385-3