Abstract
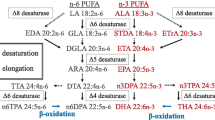
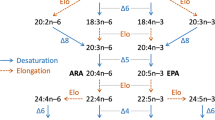
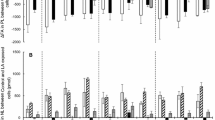
Marine fish have an absolute dietary requirement for C20 and C22 highly unsaturated fatty acids. Previous studies using cultured cell lines indicated that underlying this requirement in marine fish was either a deficiency in fatty acyl Δ5 desaturase or C18–20 elongase activity. Recent research in turbot cells found low C18–20 elongase but high Δ5 desaturase activity. In the present study, the fatty acid desaturase/elongase pathway was investigated in a cell line (SAF-1) from another carnivorous marine fish, sea bream. The metabolic conversions of a range of radiolabeled polyunsaturated fatty acids that comprised the direct substrates for Δ6 desaturase ([1-14C]18∶2n−6 and [1-14C]18∶3n−3), C18–20 elongase ([U-14C]18∶4n−3), Δ5 desaturase ([1-14C]20∶3n−6 and [1-14C]20∶5n−3), and C20–22 elongase ([1-14C]20∶4n−6 and [1-14C]20∶5n−3) were utilized. The results showed that fatty acyl Δ6 desaturase in SAF-1 cells was highly active and that C18–20 elongase and C20–22 elongase activities were substantial. A deficiency in the desaturation/elongation pathway was clearly identified at the level of the fatty acyl Δ5 desaturase, which was very low, particularly with 20∶4n−3 as substrate. In comparison, the apparent activities of Δ6 desaturase, C18–20 elongase, and C20–22 elongase were approximately 94-, 27-, and 16-fold greater than that for Δ5 desaturase toward their respective n−3 polyunsaturated fatty acid substrates. The evidence obtained in the SAF-1 cell line is consistent with the dietary requirement for C20 and C22 highly unsaturated fatty acids in the marine fish the sea bream, being primarily due to a deficiency in fatty acid Δ5 desaturase activity.
Similar content being viewed by others
Abbreviations
- AS:
-
Atlantic salmon cells
- BHT:
-
butylated hydroxytoluene
- BSA:
-
bovine serum albumin
- EFA:
-
essential fatty acid
- FAF-BSA:
-
fatty acid free-BSA
- FAME:
-
fatty acid methyl ester
- FBS:
-
fetal bovine serum
- HBSS:
-
Hank's balanced salt solution (without Ca2+ and Mg2+)
- HPTLC:
-
high-performance thin-layer chromatography
- HUFA:
-
highly unsaturated fatty acids (≥C20 with ≥3 double bonds)
- PC:
-
phosphatidylcholine
- PE:
-
phosphatidylethanolamine
- PUFA:
-
polyunsaturated fatty acid
- RTG-2:
-
rainbow trout cells
- SAF-1:
-
seam bream cell line, developed from fin tissue without immortalization
- TF:
-
turbot fin cells
- TLC:
-
thin-layer chromatography
References
Yone, Y. (1978) Essential Fatty Acids and Lipid Requirements of Marine Fish, in Dietary Lipids in Aquaculture (Jpn. Soc. Sci. Fish., eds.) pp. 43–59, Koseisha-Koseik-Abu, Tokyo, Japan.
Watanabe, T. (1982) Lipid Nutrition in Fish, Comp. Biochem. Physiol. 73B, 3–15.
Kanazawa, A. (1985) Essential Fatty Acid and Lipid Requirement of Fish, in Nutrition and Feeding of Fish (Cowey, C.B., Mackie, A.M., and Bell, J.G., eds.), pp. 281–298, Academic Press, London.
Henderson, R.J., and Tocher, D.R. (1987) The Lipid Composition and Biochemistry of Freshwater Fish, Prog. Lipid Res. 26, 281–347.
Sargent, J.R., Henderson, R.J., and Tocher, D.R. (1989) The Lipids, in Fish Nutrition (Halver, J.E., ed.), pp. 154–219, Academic Press, San Diego.
Owen, J.M., Adron, J.A., Middleton, C., and Cowey, C.B. (1975) Elongation and Desaturation of Dietary Fatty Acids in Turbot Scophthalmus maximus and Rainbow Trout Salmo gairdneri, Lipids 10, 528–531.
Cowey, C.B., Adron, J.W., Owen, J.M., and Roberts, R.J. (1976) The Effect of Different Dietary Oils on Tissue Fatty Acid and Tissue Pathology in Turbot Scophthalmus maximus, Comp. Biochem. Physiol. 53B, 399–403.
Bell, M.V., Henderson, R.J., and Sargent, J.R. (1985) Changes in the Fatty Acid Composition of Turbot (Scophthalmus maximus) in Relation to Dietary Polyunsaturated Fatty Acid Deficiencies, Comp. Biochem. Physiol. 81B, 193–198.
Tocher, D.R., Carr, J., and Sargent, J.R. (1989) Polyunsaturated Fatty Acid Metabolism in Fish Cells: Differential Metabolism of (n−3) and (n−6) Series Acids by Cultured Cells Originating from a Freshwater Teleost Fish and from a Marine Teleost Fish, Comp. Biochem. Physiol. 94B, 367–374.
Tocher, D.R., and Sargent, J.R. (1990) Effect of Temperature on the Incorporation into Phospholipid Classes and the Metabolism via Desaturation and Elongation of (n−3) and (n−6) Polyunsaturated Fatty Acids in Fish Cells in Culture, Lipids 25, 435–442.
Ghioni, C., Tocher, D.R., Bell, M.V., Dick, J.R., and Sargent, J.R. (1999) Low C18 to C20 Fatty Acid Elongase Activity and Limited Conversion of Stearidonic Acid, 18∶4n−3, to Eicosapentaenoic acid, 20∶5n−3, in a Cell Line from the Turbot, Scophthalmus maximus, Biochim. Biophys. Acta 1437, 170–181.
Mourente, G., and Tocher, D.R. (1993) Incorporation and Metabolism of 14C-Labelled Polyunsaturated Fatty Acids by Juvenile Gilthead Sea Bream Sparus aurata L. in vivo, Fish Physiol. Biochem. 10, 443–453.
Mourente, G., and Tocher, D.R. (1993) Incorporation and Metabolism of 14C-Labelled Polyunsaturated Fatty Acids in Wild-Caught Juveniles of Golden Grey Mullet, Liza aurata, in vivo, Fish. Physiol. Biochem. 12, 119–130.
Mourente, G., and Tocher, D.R. (1994) In Vivo Metabolism of [1-14C] Linolenic Acid (18∶3(n−3)) and [1-14C] Eicosapentaenoic Acid (20∶5(n−3)) in a Marine Fish: Time Course of the Desaturation/Elongation Pathway, Biochim. Biophys. Acta 1212, 109–118.
Bejar, J., Borrego, J.J., and Alvarez, M.C. (1997) A Continuous Cell Line from the Cultured Marine Fish Gilthead Seabream (Sparus aurata L.), Aquaculture 150, 143–153.
Tocher, D.R., Castell, J.D., Dick, J.R., and Sargent, J.R. (1994) Effects of Salinity on the Growth and Lipid Composition of Atlantic Salmon (Salmo salar) and Turbot (Scophthalmus maximus) Cells in Culture, Fish Physiol. Biochem. 13, 451–461.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951) Protein Measurement with Folin Phenol Reagent, J. Biol. Chem. 193, 265–275.
Folch, J., Lee, M., and Sloane-Stanley, G.H. (1957) A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues, J. Biol. Chem. 226, 497–509.
Tocher, D.R., Sargent, J.R., and Frerichs G.N. (1988) The Fatty Acid Composition of Established Fish Cell Lines After Long-Term Culture in Mammalian Sera, Fish Physiol. Biochem. 5, 219–227.
Tocher, D.R., and Harvie, D.G. (1988) Fatty Acid Compositions of the Major Phosphoglycerides from Fish Neural Tissues: (n−3) and (n−6) Polyunsaturated Fatty Acids in Rainbow Trout (Salmo gairdneri L.) and Cod (Gadus morhua) Brains and Retinas, Fish Physiol. Biochem. 5, 229–239.
Henderson, R.J., and Tocher, D.R. (1992) Thin-Layer Chromatography, in Lipid Analysis: A Practical Approach (Hamilton, R.J., and Hamilton, S. eds.), pp. 65–111, IRL Press, Oxford.
Olsen, R.E., and Henderson, R.J. (1989) The Rapid Analysis of Neutral and Polar Marine Lipids Using Double-Development HPTLC and Scanning Densitometry, J. Exp. Mar. Biol. Ecol. 129, 189–197.
Christie, W.W. (1982) Lipid Analysis, 2nd edn., Pergamon Press, Oxford.
Ghioni, C., Tocher, D.R., and Sargent, J.R. (1997) The Effect of Culture on Morphology, Lipid and Fatty Acid Composition, and Polyunsaturated Fatty Acid Metabolism of Rainbow Trout (Oncorhynchus mykiss) Skin Cells, Fish Physiol. Biochem. 16, 499–513.
Wilson, R., and Sargent, J.R. (1992) High Resolution Separation of Polyunsaturated Fatty Acids by Argentation Thin-Layer Chromatography, J. Chromatogr. 623, 403–407.
Buzzi, M., Henderson, R.J., and Sargent, J.R. (1996) The Desaturation and Elongation of Linoleic Acid and Eicosapentaenoic Acid by Hepatocytes and Liver Microsomes from Rainbow Trout (Oncorhynchus mykiss) Fed Diets Containing Fish Oil or Olive Oil, Biochim. Biophys. Acta 1299, 235–244.
Zar, J.H. (1984) in Biostatistical Analysis, Prentice-Hall, Englewood Cliffs, New Jersey.
Tocher, D.R., and Dick, J.R. (1990) Polyunsaturated Fatty Acid Metabolism in Cultured Fish Cells: Incorporation and Metabolism of (n−3) and (n−6) Series Acids by Atlantic Salmon (Salmo salar) Cells, Fish. Physiol. Biochem. 8, 311–319.
Sargent, J.R., Bell, J.G., Bell, M.V., Henderson, R.J., and Tocher, D.R. (1995) Requirement Criteria for Essential Fatty Acids, J. Appl. Ichthyol. 11, 183–198.
Sargent, J.R., Bell, M.V., Bell, J.G., Henderson, R.J., and Tocher, D.R. (1995) Origins and Functions of (n−3) Polyunsaturated Fatty Acids in Marine Organisms, in Phospholipids: Characterization, Metabolism and Novel Biological Applications (Cevc, G., and Paltauf, F., eds.), pp. 248–259, AOCS Press, Champaign.
Bell, J.G., Ghioni, C., and Sargent, J.R. (1994) Fatty Acid Composition of 10 Freshwater Invertebrates Which Are Natural Food Organisms of Atlantic Salmon (Salmo salar): A Comparison with Commercial Diets, Aquaculture 128, 301–313.
Gurr, M.I., and James, A.T. (1980) Lipid Biochemistry: An Introduction, Chapman and Hall, London.
Holman, R.T. (1986) Control of Polyunsaturated Fatty Acids in Tissue Lipids, J. Am. Coll. Nutr. 5, 183–211.
Tocher, D.R., and Sargent, J.R. (1990) Incorporation into Phospholipid Classes and Metabolism via Desaturation and Elongation of Various 14C-Labelled (n−3) and (n−6) Polyunsaturated Fatty Acids in Trout Astrocytes in Primary Culture, J. Neurochem. 54, 2118–2124.
Rivers, J.P.W., Sinclair, A.J., and Crawford, M.A. (1975) Inability of the Cat to Desaturate Essential Fatty Acids, Nature (London) 258, 171–173.
Rivers, J.P.W., Hassam, A.G., Crawford, M.A., and Brambell, M.R. (1976) The Inability of the Lion, Panthera leo, L. to Desaturate Linoleic Acid, FEBS Lett. 67, 269–270.
Hassam, A.G., Rivers, J.P.W., and Crawford, M.A. (1977) The Failure of the Cat to Desaturate Linoleic Acid; Its Nutritional Implications, Nutr. Metab. 21, 321–328.
Sinclair, A.J., McLean, J.G., and Monger E.A. (1979) Metabolism of Linoleic Acid in the Cat, Lipids 14, 932–936.
Pawlosky, J.R., Barnes, A., and Salem, N., Jr. (1994) Essential Fatty Acid Metabolism in the Feline: Relationships Between Liver and Brain Production of Long-Chain Polyunsaturated Fatty Acids, J. Lipid Res. 35, 2032–2040.
Maeda, M., Doi, O., and Akamatsu, Y. (1978) Metabolic Conversion of Polyunsaturated Fatty Acids in Mammalian Cultured Cells, Biochim. Biophys. Acta 530, 153–164.
Buttke, T.M., Cleave, S.V., Steelman, L., and McCubrey, J.A. (1989) Absence of Unsaturated Fatty Acid Synthesis in Murine T Lymphocytes, Proc. Natl. Acad. Sci. USA, 86, 6133–6137.
Rohwedder, W.K., Duval, S.M., Darhal, J.W., and Emken, E.A. (1990) Measurement of the Metabolic Interconversion of Deuterium-Labeled Fatty Acids by Gas Chromatography/Mass Spectrometry, Lipids, 25, 401–405.
Salem, N., Jr., Wegher, B., Mena, P., and Uauy, R. (1996) Arachidonic and Docosahexaenoic Acids Are Biosyntheized from Their 18-Carbon Precursors in Human Infants, Proc. Natl. Acad. Sci. USA 93, 49–54.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tocher, D.R., Ghioni, C. Fatty acid metabolism in marine fish: Low activity of fatty acyl Δ5 desaturation in gilthead sea bream (Sparus aurata) cells. Lipids 34, 433–440 (1999). https://doi.org/10.1007/s11745-999-0382-8
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11745-999-0382-8