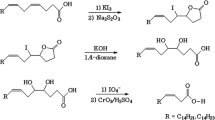
Abstract
The FA composition of 12 strains of marine aerobic anoxygenic phototrophic bacteria belonging to the genera Erythrobacter, Roseobacter, and Citromicrobium was investigated. GC-MS analyses of different types of derivatives were performed to determine the structures of the main FA present in these organisms. All the analyzed strains contained the relatively rare 11-methyloctadec-12-enoic acid, and three contained 12-methyl-octadec-11-enoic acid, which has apparently never been reported before. High amounts of the very unusual octadeca-5,11-dienoic acid were present in 9 of the 12 strains analyzed. A FA containing a furan ring was detected in three strains. Analytical data indicated that this FA was 10,13-epoxy-11-methyloctadeca-10,12-dienoic acid. A very interesting enzymatic peroxidation of the allylic carbon 10 of cis-vaccenic acid was observed in three strains. Deuterium labeling and GC-MS analyses enabled us to demonstrate that this enzymatic process involves the initial dioxygenase-mediated formation of 10-hydroperoxyoctadec-11(cis)-enoic acid, which is then isomerized to 10-hydroperoxyoctadec-11(trans)-enoic acid and converted to the corresponding hydroxy-acids and oxoacids. Different biosynthetic pathways were proposed for these different compounds.
Similar content being viewed by others
Abbreviations
- AAP:
-
aerobic anoxygenic phototrophs
- Bchl:
-
bacteriochlorophyll
- BSTFA:
-
bis(trimethylsily)trifluoroacetamide
References
Sato, K. (1978) Bacteriochlorophyll Formation by Facultative Methylotrophs, Protaminobacter rubber and Pseudomonas AM1, FEBS Lett. 85, 207–210.
Harashima, K., Shiba, T., Totsuka, T., Shimidu, U., and Taga, N. (1978) Occurrence of Bacteriochlorophyll-a in a Strain of an Aerobic Heterotrophic Bacterium, Agric. Biol. Chem. 42, 1627–1628.
Shimada, K. (1995) Aerobic Anoxygenic Phototrophs, in Anoxygenic Photosynthetic Bacteria (Blankenship, R.E., Madigan, M.T., and Bauer, C.E., eds.), p. 105–122, Kluwer Academic, Dordrecht, The Netherlands.
Yurkov, V.V., and Beatty, J.T. (1998) Aerobic Anoxygenic Phototrophic Bacteria, Microbiol. Mol. Biol. Rev. 62, 695–724.
Shiba, T., Simidu, U., and Taga, N. (1979) Distribution of Aerobic Bacteria Which Contain Bacteriochlorophyll-a, Appl. Environ. Microbiol. 38, 43–45.
Shiba, T. (1991) Roseobacter litoralis gen. nov., sp. nov. and Roseobacter denitrificans sp. nov., Aerobic Pink-Pigmented Bacteria Which Contain Bacteriochlorophyll-a, Syst. Appl. Microbiol. 14, 140–145.
Kolber, Z.S., Van Dover, C.L., Niederman, R.A., and Falkowski, P.G. (2000) Bacterial Photosynthesis in Surface Waters of the Open Ocean, Nature 407, 177–179.
Kolber, Z.S., Plumley, F.G., Lang, A.S., Beatty, J.T., Blankenship, R.E., VanDover, C.L., Vetriani, C., Koblizek, M., Rathgeber, C., and Falkowski, P.G. (2001) Contribution of Aerobic Photoheterotrophic Bacteria to the Carbon Cycle in the Ocean, Science 292, 2492–2495.
Goericke, R. (2002) Bacteriochlorophyll-a in the Ocean: Is Anoxygenic Bacterial Photosynthesis Important? Limnol. Oceanogr. 47, 290–295.
Koblízek, M., Falkowski, P.G., and Kolber, Z.S. (2005) Diversity and Distribution of Photosynthetic Bacteria in the Black Sea, Deep Sea Res. II, in press.
Koblízek, M., Ston-Egiert, J., Sagan, S., and Kolber, Z. (2004) Diel Changes in Bacteriochlorophyll-a Concentration Suggest Rapid Bacterioplankton Cycling in the Baltic Sea, FEMS Microbiol Ecol. 51, 353–361.
Shiba, T., and Simidu, U. (1982) Erythrobacter longus gen. nov., sp. nov., an Aerobic Bacterium Which Contains Bacteriochlorophyll-a, Int. J. Syst. Bacteriol. 32, 211–217.
Nishimura, Y., Muroga, Y., Saito, S., Shiba, T., Takamiya, K., and Shioi, Y. (1994) DNA Relatedness and Chemotaxonomic Feature of Aerobic Bacteriochlorophyll-Containing Bacteria Isolated from Coasts of Australia, J. Gen. Appl. Microbiol. 40, 287–296.
Suzuki, T., Muroga, Y., Takahama, M., and Nishimura, Y. (2000) Roseibium denhamense gen. nov., sp. nov. and Roseibium hamelinense sp. nov., Aerobic Bacteriochlorophyll-Containing Bacteria Isolated from the East and West Coasts of Australia, Int. J. Syst. Bacteriol. 50, 2151–2156.
Koblizek, M., Béjà, O., Bidigare, R.R., Christensen, S., Benetiz-Nelson, B., Vetriani, C., Kolber, M.K., and Falkowski, P.G. (2003) Isolation and Characterization of Erythrobacter sp. Strains from the Upper Ocean, Arch. Microbiol. 180, 327–338.
Volkman, J.K., Farmer, C.L., Barrett, S.M., and Sikes, E.L. (1997) Unusual Dihydroxysterols as Chemotaxonomic Markers for Microalgae from the Order Pavlovales (Haptophyceae), J. Phycol. 33, 1016–1023.
Marchand, D., and Rontani, J.-F. (2003) Visible Light-Induced Oxidation of Lipid Components of Purple Sulphur Bacteria: A Significant Process in Microbial Mats, Org. Geochem. 34, 61–79.
Mihara, S., and Tateba, H. (1986) Photosensitized Oxygenation Reactions of Phytol and Its Derivatives, J. Org. Chem. 51, 1142–1144.
Harwood, J.L., and Russell, N.L. (1984) Lipids in Plants and Microbes, pp. 1–162, Allen & Unwin, London.
Wilkinson, S.G. (1988) Gram-Negative Bacteria, in Microbial Lipids (Ratledge, C., and Wilkinson, S.G., eds.), pp. 299–488, Alden Press, London.
Rontani, J.-F., and Aubert, C. (2003) Electron Ionization Mass Spectral Fragmentation of C19 Isoprenoid Aldehydes and Carboxylic Acid Methyl and Trimethylsilyl Esters, Rapid Commun. Mass Spectrom. 17, 949–956.
McCloskey, J.A., and McClelland, M.J. (1965) Mass Spectra of O-Isopropylidene Derivatives of Unsaturated Fatty Esters, J. Am. Chem. Soc. 87, 5090–5093.
Andersson, B.A., Christie, W.W., and Holman, R.T. (1974) Mass Spectrometric Determination of Positions of Double Bonds in Polyunsaturated Fatty Acid Pyrrolidides, Lipids 10, 215–219.
Capella, P., and Zorzut, C.M. (1968) Determination of Double Bond Position in Monounsaturated Fatty Acid Esters by Mass Spectrometry of Their Trimethylsilyloxy Derivatives, Anal. Chem. 40, 1458–1463.
de Leeuw, J.W., van der Meer, J.W., Rijpstra, W.I.C., and Schenck, P.A. (1980) On the Occurrence and Structural Identification of Long Chain Ketones and Hydrocarbons in Sediments, in Advances in Organic Geochemistry 1979 (Douglas, A.G., and Maxwell, J.R., eds.), pp. 211–217, Pergamon Press, Oxford.
Andersson, B.A., and Holman, R.T. (1975) Mass Spectrometric Localization of Methyl Branching in Fatty Acids Using Acylpyrrolidines, Lipids 10, 716–718.
Couderc, F. (1995) Gas Chromatography/Tandem Mass Spectrometry as an Analytical Tool for the Identification of Fatty Acids, Lipids 30, 691–699.
Shirasaka, N., Nishi, K., and Shimizu, S. (1997) Biosynthesis of Furan Fatty Acids (F-acids) by a Marine Bacterium, Shewanella putrefaciens, Biochim. Biophys. Acta 1346, 253–260.
Kerger, B.D., Nichols, P.D., Antworth, C.P., Sand, W., Bock, E., Cox, J.C., Langworthy, T.A., and White, D.C. (1986) Signature Fatty Acids in the Polar Lipids of Acid-Producing Thiobacillus spp.: Methoxy, Cyclopropyl α-Hydroxy-Cyclopropyl and Branched and Normal Monoenoic Fatty Acids, FEMS Microbiol. Ecol 38, 67–77.
Wolff, R.L., Christie, W.W., and Marpeau, A.M. (1999) Reinvestigation of the Polymethylene-Interrupted 18∶2 and 20∶2 Acids of Ginkgo biloba Seed Lipids, J. Oil Am. Chem. Soc. 76, 273–277.
Volkman, J.K., Jeffrey, S.W.J., Nichols, P.D., Rogers, G.I., and Garland, C.D. (1989) Fatty Acid and Lipid Composition of 10 Species of Microalgae Used in Mariculture, J. Exp. Mar. Biol. Ecol. 128, 219–240.
Viso, A.-C., and Marty, J.-C. (1993) Fatty Acids from 28 Marine Microalgae, Phytochemistry 34, 1521–1533.
Volkman, J.K., Barrett, S.M., Blackburn, S.I., Mansour, M.P., Sikes, E.L., and Gelin, F. (1998) Microalgal Biomarkers: A Review of Recent Research Developments, Org. Geochem. 29, 1163–1179.
Reuss, N., and Poulsen, L.K. (2002) Evaluation of Fatty Acids as Biomarkers for a Natural Plankton Community. A Field Study of a Spring Bloom and a Post-bloom Period of West Greenland, Mar. Biol. 141, 423–434.
Imhoff, J.F., and Bias-Imhoff, U. (1995) Lipids, Quinones and Fatty Acids of Anoxygenic Phototrophic Bacteria, in Anoxygenic Photosynthetic Bacteria (Blankenship, R.E., Madigan, M.T. and Bauer, C.E., eds.), pp. 179–205, Kluwer Academic, Dordrecht, The Netherlands.
McCloskey, J.A. (1969) Mass Spectrometry of Lipids and Steroids, Methods Enzymol. 14, 382–450.
Shirasaka, N., Nishi, K., and Shimizu, S. (1995) Occurrence of a Furan Fatty Acid in Marine Bacteria, Biochim. Biophys. Acta 1258, 225–227.
Scheinkönig, J., Hannemann, K., and Spiteller, G. (1995) Methylation of the β-Positions of the Furan Ring in F-acids, Biochim. Biophys. Acta 1254, 73–76.
Busse, H.J., Kämpfer, P., and Denner, E.B.M. (1999) Chemotaxonomic Characterization of Sphingomonas, J. Ind. Microbiol. Biotechnol. 23, 242–251.
Rontani, J.-F., Koblizek, M., Beker, B., Bonin, P., and Kolber, Z. (2003) On the Origin of cis-Vaccenic Acid Photodegradation Products in the Marine Environment, Lipids 38, 1085–1092.
Klok, J., Baas, M., Cox, H.C., de Leeuw, J.W., Rijpstra, W.I.C., and Schenck, P.A. (1988) The Mode of Occurrence of Lipids in a Namibian Shelf Diatomaceous Ooze with Emphasis on the β-Hydroxy Fatty Acids, Org. Geochem. 12, 75–80.
Marchand, D., Grossi, V., Hirschler-Réa, A., and Rontani, J.-F. (2002) Regiospecific Enzymatic Oxygenation of cis-Vaccenic Acid During Aerobic Senescence of the Halophilic Purple Sulfur Bacterium Thiohalocapsa halophila Lipids 37, 541–548.
Kühn, H., Schewe, T., and Rapoport, S.M. (1986) The Stereochemistry of the Reactions of Lipoxygenases and Their Metabolites. Proposed Nomenclature of Lipoxygenases and Related Enzymes, Adv. Enzymol. 58, 273–311.
Wang, T., Yu, W.G., and Powell, W.S. (1992) Formation of Monohydroxy Derivatives of Arachidonic Acid, Linoleic Acid, and Oleic Acid During Oxidation of Low Density Lipoprotein by Copper Ions and Endothelial Cells, J. Lipid Res. 33, 525–537.
Guerrero, A., Casals, I., Busquets, M., Leon, Y., and Manresa, A. (1997) Oxidation of Oleic Acid to (E)-10-Hydroperoxy-8-octadecenoic and (E)-10-Hydroxy-8-octadecenoic Acids by Pseudomonas sp. 42A2, Biochim. Biophys. Acta 1347, 75–81.
Oliw, E.H., Su, C., Skogstrom, T., and Benthin, G. (1998) Analysis of Novel Hydroperoxides and Other Metabolites of Oleic, Linoleic, and Linolenic Acids by Liquid Chromatography-Mass Spectrometry, Lipids 33, 843–852.
Clapp, C.H., Senchak, S.E., Stover, T.J., Potter, T.C., Findeis, P.M., and Novak, M.J. (2001) Soybean Lipoxygenase-Mediated Oxygenation of Monounsaturated Fatty Acids to Enones, J. Am. Chem. Soc. 123, 747–748.
Schewe, T., Rapoport, S.M., and Kühn, H. (1986) Enzymology and Physiology of Reticulocyte Lipoxygenase: Comparison with Other Lipoxygenases, Adv. Enzymol. 58, 191–272.
Marchand, D., and Rontani, J.-F. (2001) Characterisation of Photooxidation and Autoxidation Products of Phytoplanktonic Monounsaturated Fatty Acids in Marine Particulate Matter and Recent Sediments, Org. Geochem. 32, 287–304.
Galliard, T., and Chan, H.W.-S. (1980) Lipoxygenases, in The Biochemistry of Plants: A Comprehensive Treatise (Stumpf, P.K., and Conn, E.E., eds.), Vol. 4, pp. 131–161, Academic Press, New York.
Hamberg, M. (1987) Mechanism of Corn Hydroperoxide Isomerase: Detection of 12,13(S)-Oxido-9(Z),11-octadecadienoic Acid, Biochim. Biophys. Acta 920, 76–84.
Galliard, T., Matthew, J.A., Fishwick, M.J., and Wright, A.J. (1976) The Enzymatic Degradation of Lipids Resulting from Physical Disruption of Cucumber (Cucumis sativus) Fruit, Phytochemistry 15, 1647–1650.
Porter, N.A., Caldwell, S.E., and Mills, K.A. (1995) Mechanisms of Free Radical Oxidation of Unsaturated Lipids, Lipids 30, 277–290.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rontani, J.F., Christodoulou, S. & Koblizek, M. GC-MS structural characterization of fatty acids from marine aerobic anoxygenic phototrophic bacteria. Lipids 40, 97–108 (2005). https://doi.org/10.1007/s11745-005-1364-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-005-1364-6