Abstract
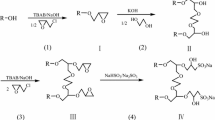
In the search for environmentally safe surfactants made from inexpensive and renewable sources, the interest has mainly been focused on new saccharide derivatives. This report describes the synthesis of newly designed nonionic gemini compounds comprising two reduced sugar headgroups, two alkyl tails, and a 1,1′-ethylenebisurea entity as the spacer linking two amphiphilic glucose-derived moieties. Thus, the series of N,N′-bis[(3-alkyl-3-deoxy-d-glucitol)ureido]ethylenediamines (bis(CnGT), with Cn=n-C9H9, n-C6-H13, n-C8H17, n-C10H21, or n-C12H25), were prepared using a convenient procedure starting from easily accessible reagents such as d-glucose, n-alkylamines, urea, and ethylenediamine. Their structure and purity were confirmed by means of elemental analysis, electrospray ionization mass spectrometry, and 1H and 13C nuclear magnetic resonance spectroscopy. Additionally, the present contribution introduces selected properties of these surfactants, including their thermotropic behavior and biological properties. The presence of two phase transition points, determined using the differential scanning calorimetry method, indicates liquid-crystalline mesophase formation upon heating. Furthermore, using the closed-bottle test (OECD Guideline 301D) as well as the biological oxygen demand test for insoluble substances for biodegradability measurements, it has been concluded that the tested glucose-derived gemini structures achieve more than 60% biodegradation after 64–75 test days. All tested surfactants were practically nontoxic to bacteria, yeast, and molds. Owing to their fitting aggregation ability as well as their nontoxicity, they constitute an interesting group of surfactants for various applications.
Similar content being viewed by others
Abbreviations
- bis(CnCT):
-
N,N′-bis[3-alkyl-3-deoxy-D-glucitol) ureido]ethylenediamines
- BOD:
-
biological oxygen demand
- BODIS:
-
biological oxygen demand test for insoluble substances
- cfu:
-
colony forming units
- DSC:
-
differential scanning calorimetry
- ESI-MS:
-
electrospray ionization mass spectrometry
- MIC:
-
minimal inhibitory concentration
- NMR:
-
nuclear magnetic resonance
- PCM:
-
Polish Collection of Microorganisms
- TOD:
-
theoretical oxygen demand
References
Luk, Y.-Y., and N.L. Abbott, Application of Functional Surfactants, Curr. Opin. Colloid Interface Sci. 7:267 (2002).
Chevalier, Y., New Surfactants: New Chemical Functions and Molecular Architectures, Curr. Opin. Colloid Interface Sci. 7:3 (2002).
Söderman, O., and I. Johansson, Polyhydroxyl-Based Surfactants and Their Physico-Chemical Properties and Applications, Curr. Opin. Colloid Interface Sci. 4:391 (2000).
Stubenrauch, C., Sugar surfactants-Aggregation, Interfacial, and Adsorption Phenomena, Curr. Opin. Colloid Interface Sci. 6:160 (2001).
Burczyk, B., Novel Saccharide-Based Surfactants, in Novel Surfactants. Preparation, Applications, and Biodegradability, 2nd ed., edited by K. Holmberg, Marcel Dekker, New York, 2003, p. 129–192.
Maliszewska, I., K.A. Wilk, B. Burczyk, and L. Syper, Antimicrobial Activity and Biodegradability of N-Akylaldonamides, Progr. Colloid Polym. Sci. 118:172 (2001).
Wilk, K.A., L., Syper, B. Burczyk, I. Maliszewska, M. Jon, and B.W. Domagalska, Preparation and Properties of New Lactose-Based Surfactants, J. Surfact. Deterg. 4:155 (2001).
Syper, L., K.A. Wilk, A. SokoÁowski, and B. Burczyk, Synthesis and Surface Properties of N-Alkylaldonamides, Progr. Colloid Polym. Sci. 110:199 (1998).
Burczyk, B., L. Syper, and K.A. Wilk, New Surface Active Aldonamides and Their Preparation, Polish Patent Application P. 331294, 1999.
Burczyk, B., K.A. Wilk, A. SokoÁowski, and L. Syper, Synthesis and Surface Properties of N-Alkyl-N-methylgluconamides and N-Alkyl-N-methyllactobionamides, J. Colloid Interface Sci. 240:552 (2001).
Wilk, K.A., L. Syper, B. Burczyk, A. SokoÁowski, and B.W. Domagalska, Synthesis and Surface Properties of New Dicephalic Saccharide-Derived Surfactants, J. Surfact. Deterg. 3: 185 (2000).
Eastoe, J., P. Rogueda, B.J. Harrison, A.M. Howe, and A.R. Pitt, Properties of a Dichained “Sugar Surfactant”, Langmuir 10:4429 (1994).
Eastoe, J., P. Rogueda, A.M. Howe, A.R. Pitt, and R.K. Heenan, Properties of New Glucamide Surfactants, Langmuir 12:2701 (1996).
Briggs, C.B.A., I.M. Newington, and A.R. Pitt, Synthesis and Properties of Some Novel Nonionic Polyol Surfactants, J. Chem. Soc., Chem. Commun. 3:379 (1995).
Kjellin, U.R.M., P.M. Claesson, and E.N. Vulfson, Studies of N-Dodecyllactobionamide, Maltose 6′-O-Dodecanoate, and Octyl-β-glucoside with Surface Tension, Surface Force, and Wetting Technique, Langmuir 17:1941 (2001).
Claesson, P.M., and U.R.M. Kjellin, Sugar Surfactans, in Encyclopedia of Surface and Colloid Science, Marcel Dekker New York, 2002, p. 4909–4925.
Rico-Lattes, I., and A. Lattes, Synthesis of New Sugar-Based Surfactants Having Biological Applications: Key Role of Their Self-Association, Colloids Surf. A 123–124:37 (1997).
Castro, M.J.L., J. Kovensky, and A.F. Girelli, Gemini Surfactants from Alkyl Glucosides, Tetrahedron Lett. 38:3995 (1997).
Castro, M.J.L., J. Kovensky, and A.F. Girelli, New Dimeric Surfactants from Alkyl Glucosides, Tetrahedron 55:12711 (1999).
Gao, C., A. Millqvist-Fureby, M.J. Whitcombe, and E.N. Vulfson, Regioselective Synthesis of Dimeric (gemini) and Trimeric Sugar-Based Surfactants, J. Surfact. Deterg. 2:293 (1999).
Gao, C., M.J. Whitcombe, and E.N. Vulfson, Enzymatic Synthesis of Dimeric and Trimeric Sugar-Fatty Acid Esters, Enzyme Microb. Technol. 25:264 (1999).
Wilk, K.A., L. Syper, B.W. Domagalska, U. Laska, I. Maliszewska, and R. Gancarz, Aldonamide-Type Gemini Surfactants: Synthesis, Structural Analysis and Biological Properties, J. Surfact. Deterg. 5:235 (2002).
Laska, U., and K.A. Wilk, Surface and Micellar Properties of New Nonionic Gemini Aldonamide-Type Surfactants, J. Colloid Interface Sci. 271:206 (2004).
Fielden, M.L., C. Perrin, A. Kremer, M. Bergsma, M.C. Stuart, P. Camilleri, and J.B.F.N., Engberts, Sugar-Based Tertiary Amino Gemini Surfactants with a Vesicle-to-Micelle Transition in the Endosomal pH Range Mediate Efficient Transfection in vitro, Eur. J. Biochem. 268:1269 (2001).
Johnsson, M., A. Wagenaar, and J.B.F.N. Engberts, Sugar-Based Gemini Surfactants with a Vesicle-to-Micelle Transition at Acidic pH and a Reversible Vesicle Flocculation Near Neutral pH, J. Am. Chem. Soc. 125:757 (2003).
van Doren, H.A., E. Smits, J.M. Pestman, J.B.F.N. Engberts, and R.M. Kellogg, Mesogenic Sugars. From Aldoses to Liquid Crystals and Surfactants, Chem. Soc. Rev. 29:175 (2000).
Syper, L., K.A. Wilk, and B. Matuszweska, Preparation Method for Nonionic Sugar Surfactants, Polish Patent Application P-347346, 2001.
Syper, L., K.A. Wilk, and B. Matuszewska, Preparation Method for Bisalkonoylbisglucamines, Polish Patent Application P347345, 2001.
Pestman, J.M., K.R. Terpstra, M.C.A. Stuart, H.A. van Doren, A. Brisson, R.M. Kellogg, and J.B.F.N. Engberts, Nonionic Bolaamphiphiles and Gemini Surfactants Based on Carbohydrates, Langmuir 13:6857 (1997).
Begsma, M., M.L. Fielden, and J.B.N.F. Engberts, pH-Dependent Aggregation Behavior of a Sugar-Amine Gemini Surfactant in Water: Vesicles, Micelles, and Monolayers of Hexane-1,6-bis(hyxadecyl-1′-deoxyglucitylamine), J. Colloid Interface Sci. 243:491 (2001).
van Eijk, M.C.P., M. Bergsma, and S.-J. Marrink, Association Behaviour of Glucitol Amine Gemini Surfactants. Self-Consistent-Field Theory and Molecular-Dynamics Simulations, Eur. Phys. J. E 7:317 (2002).
Gronwald, O., E. Snip, and S. Shinkai, Gelators for Organic Liquids Based on Self-Assembly: A New Facet of Supramolecular and Combinatorial Chemistry, Curr. Opin. Colloid Interface Sci. 7:148 (2002).
Bachmann, W.E., W.J. Horton, E.L. Jenner, N.W. MacNaughton, and C.E. Maxwell, The Nitration of Derivatives of Ethylenediamine, J. Am. Chem. Soc. 72:3132 (1950).
Bristline, R.G., E.W. Maurer, F.D. Smith, and W.M. Linfield, Fatty Acid Amides and Anilides, Synthesis and Antimicrobial Properties, J. Am. Oil Chem. Soc. 57:98 (1980).
Organization for Economic Cooperation and Development, Guidelines for Testing Chemicals: Closed-Bottle Test No. 301D, 1981.
Richterich, K., H. Berger, and J. Steber, Determination of “Ready Biodegradability” of Poorly Soluble Compounds with the “Two-Phase Closed Bottle Test”, Henkel Referate 35:47 (1999).
Ginkel, C.G., and C.A. Stroo, Simple Method to Prolong the Closed Bottle Test for the Determination of the Inherent Biodegradability, Ecotox Environ. Saf. 24:319 (1992).
Tundo, P., P. Anastas, D. StC. Black, J. Breen, T. Collins, S. Memoli, J. Miyamoto, M. Polyakoff, and W. Tumas, Synthetic Pathways and Processes in Green Chemistry. Introductory overview, Pure Appl. Chem. 72:1207 (2000).
Retailleau, L., A. Laplace, H. Fensterbank, and C. Larpent, Synthesis, Structural Analysis, and Properties of N-Alkylglucosyl (meth)acrylamides: New Reactive Sugar Surfactants, J. Org. Chem. 63:608 (1998).
Zhang, T., and R.E. Marchant, Novel Polysaccharide Surfactants: The Effect of Hydrophobic and Hydrophilic Chain Length on Surface Active Properties, J. Colloid Interface Sci. 177:419 (1996).
Coppola, L., A. Gordano, A. Procopio, and G. Sindona, Phase Equilibria and Physical-Chemical Properties of Sugar-Based Surfactants in Aqueous Solutions, Colloids Surf. A 196:175 (2002).
Jeffrey, G.A., and L.A. Wingert, Carbohydrate Liquid Crystals, Liq. Cryst. 12:179 (1992).
van Doren, H.A., and K.R. Terpstra, Structure-Property Relationships in D-Glucitol Derivatives with Two Geminal Hydrocarbon Chains, J. Mater. Chem. 5:2153 (1995).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Laska, U., Wilk, A., Maliszewska, I. et al. Novel glucose-derived gemini surfactants with a 1,1′-ethylenebisurea spacer: Preparation, thermotropic behavior, and biological properties. J Surfact Deterg 9, 115–124 (2006). https://doi.org/10.1007/s11743-006-0380-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11743-006-0380-0