Abstract
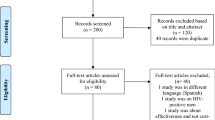
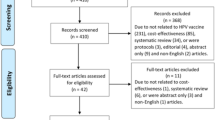
The human papilloma virus (HPV) vaccine is a new and expensive vaccine potentially effective in the prevention of a cancer. We reviewed the economic evaluations (EEs) on the vaccine in the EU to assess their potential contribution to public decision-making in a fairly homogeneous setting where HPV vaccination has been widely adopted. A literature search on PubMed selected EEs on HPV vaccines in the EU for the period 2007–2010 using the terms “HPV vaccines” and “Costs and cost analysis.” Fifteen articles were eventually selected. All studies were based on modelling techniques, either “cohort” or “dynamic transmission”: three were cost utility, three cost-effectiveness, and the remainder included both. The ten studies explicitly assessing one of the two vaccines were all sponsored by their manufacturer, while the five studies unrelated to the vaccine type were funded by public agencies. Apart from two studies, utility estimates were always obtained from three US sources. Direct costs were always vaccination, diagnosis and treatment of related pathologies. Incremental cost-effectiveness ratio (ICER) results were less favourable when life years gained were valued rather than quality-adjusted life years, genital warts were excluded, and booster doses and extension of vaccination to men were included in the base-case analysis. All but one of the sponsored EEs recommend in favour of the vaccination strategy, which is dominant in one English study. The ICER results were very sensitive to discount rates, followed by duration of protection and vaccine price. At such an early stage, when the vaccines’ efficacy have been demonstrated by well-designed studies, it is not possible (and not even reasonable) to wait for several years to measure their effectiveness; public decision-makers might benefit more from EEs designed to indicate sustainable prices using realistic estimates of crucial variables like coverage rates, rather than referring to a large number of assumptions in order to show acceptable cost-effectiveness.

Similar content being viewed by others
References
Bosch FX, Lorincz A, Muñoz N et al (2002) The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55:244–265
Pagliusi SR, Aguado MT (2004) Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 23:569–578
Myers E, Huh WK, Wright JD et al (2008) The current and the future role of screening in the era of HPV vaccination. Gynecol Oncol 109(2 Suppl):S31–S39
WHO (2008) Preparing for the introduction of HPV Vaccine in the WHO European Region: strategy paper. Vaccine-Preventable Diseases and Immunization Programme. World Health Organization, Copenhagen
Anttila A, von Karsa L, Aasmaa A et al (2009) Cervical cancer screening policies and coverage in Europe. Eur J Cancer 45:2649–2658
Bosch FX, Castellsagué X, de Sanjosé S (2008) HPV and cervical cancer: screening or vaccination? Br J Cancer 98:15–21
Kim S-Y, Goldie SJ (2008) Cost-effectiveness analyses of vaccination programmes, a focused review of modelling approaches. Pharmacoeconomics 26(3):191–215
Marra F, Cloutier K, Oteng B et al (2009) Effectiveness and cost effectiveness of human papillomavirus vaccine. A systematic review. Pharmacoeconomics 27:127–147
Cuschieri K (2009) Should boys receive the human papillomavirus vaccine? No. BMJ 339:b4921
Techakehakij W, Feldman RD (2008) Cost-effectiveness of HPV vaccination compared with Pap smear screening on a national scale: a literature review. Vaccine 28:6258–6265
Ortega-Sanchez IR, Lee GM, Jacobs RJ et al (2008) Projected cost-effectiveness of new vaccines for adolescents in the United States. Pediatrics 121(Suppl 1):563–578
Newal AT, Beutels P, Wood JG et al (2007) Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 7:289–296
Schiffman M, Castle PE, Jeronimo J et al (2007) Human papillomavirus and cervical cancer. Lancet 370:890–907
Bonati M, Garattini S (2009) Controlling cervical cancer. Pharmacoeconomics 27(2):91–93
Garland SM, Hernandez-Avila M, Wheeler CM et al (2007) Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 356:1928–1943
La Torre G, de Waure C, Chiaradia G, Mannocci A, Ricciardi W (2010) HPV vaccine eficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine 25:8352–8358
De Pouvourville G, Ulmann P, Nixon J et al (2005) The diffusion of health economics knowledge in Europe. The EURONHEED (European Network of Health Economic Evaluation Databases) Project. Pharmacoeconomics 23(2):113–120
Usher C, Tilson L, Olsen J et al (2008) Cost-effectiveness of human papillomavirus vaccine in reducing the risk of cervical cancer in Ireland due to HPV types 16 and 18 using a transmission dynamic model. Vaccine 26(44):5654–5661
Goldie SJ, Goldhaber-Fiebert JD, Garnett GP (2006) Chapter 18: Public health policy for cervical cancer prevention: The role of decision science, economic evaluation, and mathematical modeling. Vaccine 24(Suppl 3):S155–S163
La Torre G, de Waure C, Chiaradia G et al (2010) The health technology assessment of bivalent HPV vaccine Cervarix® in Italy. Vaccine 28:3379–3384
De Kok IM, van Ballegooijen M, Habbema JDF (2009) Cost-effectiveness analysis of human papillomavirus vaccination in The Netherlands. J Natl Cancer Inst 101(15):1083–1092
Zechmeister I, de Blasio BF, Garnett G et al (2009) Cost-effectiveness analysis of human papillomavirus—vaccination programs to prevent cervical cancer in Austria. Vaccine 27(37):5133–5141
Rogoza RM, Westra TA, Ferko N et al (2009) Cost-effectiveness analysis of prophylactic vaccination against human papillomavirus 16/18 for the prevention of cervical cancer: adaptation of an existing cohort model to the situation in The Netherlands. Vaccine 27(35):4776–4783
Thiry N, De Laet C, Hulstaert F et al (2009) Cost-effectiveness of human papillomavirus vaccination in Belgium: do not forget about cervical cancer screening. Int J Technol Assess Health Care 25(2):161–170
Annemans L, Rémy V, Oyee J et al (2009) Cost-effectiveness evaluation of a quadrivalent human papillomavirus vaccine in Belgium. Pharmacoeconomics 27(3):231–245
Mennini FS, Giorgi Rossi P, Palazzo F et al (2009) Health and economic impact associated with a quadrivalent HPV vaccine in Italy. GynecolOncol 112(2):370–376
Coupé VMH, van Ginkel J, de Melker HE et al (2009) HPV vaccination to prevent cervical cancer in The Netherlands: model-based cost-effectiveness. Int J Cancer 124(4):970–978
Jit M, Choi YH, Edmunds WJ (2008) Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 337:769. doi:10.11367bmj.a769
Dasbach EJ, Insinga RP, Elbasha EH (2008) The epidemiological and economic impact of a quadrivalent human papillomavirus vaccine (6/11/16/18) in the UK. BJOG 115(8):947–956
Bergeron C, Largeron N, McAllister R et al (2008) Cost-effectiveness analysis of the introduction of a quadrivalent human papilloma vaccine in France. Int J Technol Assess Health Care 24(1):10–19
Boot HJ, Wallenburg I, de Melker HE et al (2007) Assessing the introduction of universal human papillomavirus vaccination for preadolescent girls in The Netherlands. Vaccine 25(33):6245–6256
Kulasingam SL, Benard S, Barnabas RV et al (2008) Adding a quadrivalent human papillomavirus vaccine to the UK cervical cancer screening programme: a cost-effectiveness analysis. Cost Eff Resour Alloc 6(4):4–15
Hillemans P, Petry KU, Largeron N et al (2009) Cost-effectiveness of a tetravalent human papillomavirus vaccine in Germany. J Public Health 17:77–86
Olsen J, Jepsen MR (2010) Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. Int J Technol Assess Health Care 26(2):183–191
Villa LL, Perez G, Kjaer SK et al (2007) Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 356:1915–1927
Paavonen J, Naud P, Salmeròn J et al (2009) Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314
Harper DM, Franco EL, Wheeler CM et al (2006) Sustained efficacy up to 4–5 years of a bivalent L1 virus like particle vaccine against human papillomavirus 16 and 18: follow-up from a randomised control trial. Lancet 367:1247–1255
Villa LL, Costa RLR, Petta CA et al (2006) High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1-like-particle vaccine through 5 years of follow-up. Br J Cancer 95:1459–1466
Harper DM, Franco EL, Wheeler C et al (2004) Efficacy of bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757–1765
Villa LL, Costa RLR, Petta CA et al (2005) Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 6:271–278
Stoykova B, Dowie R (2007) HPV testing matters—findings from a time trade-off survey in England. In: IHEA 2007 6th World Congress: explorations in health economics paper. Available at SSRN http://ssrn.com/abstract=945729
Elbasha EH, Dasbach EJ, Insinga RP (2007) Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 13:28–41
Myers ER, Green S, Lipkus I (2004) Patient preferences for health states related to HPV infection: visual analogue scales vs. time trade-off elicitation. In: Proceedings of the 21st international papillomavirus conference. Mexico City, Mexico, 20–26 February
Gold M, Franks P, McCoy KI et al (1998) Towards consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care 36:778–792
Woodman CBJ, Collins SI, Young LS (2007) The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer 7:11–22
Wolstenholme JL, Whynes DK (1998) Stage-specific treatment costs for cervical cancer in the United Kingdom. Eur J Cancer 34(12):1889–1893
Oostenbrink JB, Bouwmans CA, Koopmanschap MA et al (2004) Richtlijnen voor Kostenschattingen in de Gesondheidszorg Guideline for costinng research, methods and standardized prices for economic evaluations in health care. Health Care Insurance Board, Diemen (Netherlands)
Cleemput I, Van Wilder P, Vrijens F et al (2008) Guidelines for pharmaco-economic evaluations in Belgium. Health Technology Assessment (HTA). In: F.K.v.d.G. (KCE) (ed) KCE reports. Belgian Health Care Knowledge Centre (KCE), Brussels
National Institute for Health and Clinical Excellence (2004) Guide to the methods of technology appraisal (reference N0515). http://www.nice.org.uk/niceMedia/pdf/TAP_Methods.pdf
Haug CJ (2008) Human papillomavirus vaccination—reasons for caution. N Engl J Med 359(8):861–862
Loos AH, Bray F, McCarron P et al (2004) Sheep and goats: separating cervix and corpus uteri from imprecisely coded uterine cancer deaths, for studies of geographical and temporal variations in mortality. Eur J Cancer 40:2794–2803
Knies S, Evers SMAA, Candel MJJM et al (2009) Utilities of the EQ-5D. Transferable or not? Pharmacoeconomics 27(9):767–779
Korfage IJ, Essink-Bot ML, Mols F et al (2009) Health-related quality of life in cervical cancer survivors: a population-based survey. Int J Radiat Oncol Biol Phys 73(5):1501–1509
Whynes DK, Woolley C, Philips Z, for the TOMBOLA Group (2008) Management of low-grade cervical abnormalities detected at screening: which method do women prefer? Cytopathology 19:355–362
Bell CM, Urbach DR, Ray JG et al (2006) Bias in published cost effectiveness studies: systematic review. BMJ 332:699–703
Puig-Junoy J, Lopez-Valcarcel BG (2009) Economic evaluations of massive HPV vaccination: within-study and between study variations in incremental cost per QALY gained. Prev Med 48:444–448
Acknowledgments
No funding to declare.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koleva, D., De Compadri, P., Padula, A. et al. Economic evaluation of human papilloma virus vaccination in the European Union: a critical review. Intern Emerg Med 6, 163–174 (2011). https://doi.org/10.1007/s11739-011-0529-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-011-0529-3