Abstract
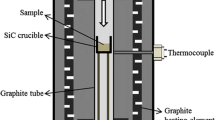
Quartz and carbonaceous materials, which are used in the production of silicon as well as electrodes and refractories in the silicon furnace, contain trace elements mostly in the form of oxides. These oxides can be reduced to gaseous compounds and leave the furnace or stay in the reaction products—metal and slag. This article examines the behavior of trace elements in hydrothermal quartz and quartzite in the reaction of SiO2 with Si or SiC. Mixtures of SiO2 (quartz or quartzite), SiC, and Si in forms of lumps or pellets were heated to 1923 K and 2123 K (1650°C and 1850°C) in high purity graphite crucibles under Argon gas flow. The gaseous compounds condensed in the inner lining of the tube attached to the crucible. The phases present in the reacted charge and the collected condensates were studied quantitatively by X-ray diffraction (XRD) and qualitatively by Electron Probe Micro Analyzer (EPMA). Contaminants in the charge materials, reacted charge and condensate were analyzed by Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS). Muscovite in the mineral phase of quartz melted and formed two immiscible liquid phases: an Al-rich melt at the core of the mineral, and a SiO2-rich melt at the mineral boundaries. B, Mn, and Pb in quartz were removed during heating in reducing atmosphere at temperature above 1923 K (1650°C). Mn, Fe, Al and B diffused from quartz into silicon. P concentration was under the detection limit. Quartzite and hydrothermal quartz had different initial impurity levels: quartzite remained more impure after reduction experiment but approached purity of hydrothermal quartz upon silica reduction.





Similar content being viewed by others
References
Panel Discussion: Crystal Clear in the 6th Framework of Program of EU. Amsterdam, 2008.
H. Yao, H. Minato, I.S.N. Mkilaha, and I. Naruse: J. Jpn. I. Met., 2001, pp. 256–62.
Q. Wang, J. Qiu, Y. Liu, and C. Zheng: Fuel Process. Technol., 2004. 85: p. 1431-1441.
M. Garcia and E.H. Myrhaug: Revisjon av materialbalanse for sporelementer i Si-ovn basert på målekampanje på Elkem Thamshavn April 2007. Elkem Silicon, Oslo, 2007.
E.H. Myrhaug and H. Tveit: Electric Furnace Conference Proceedings, vol. 58. AIST, Warrendale, PA, 2000, pp. 591–04.
R. Kvande, L. Nygaard, S. Stute, and P.C.P. Bronsveld: 25th European Photovoltaic Solar Energy Conference and Exhibition/Conference on Photovoltaic Energy Conversion, Valencia, Spain, 2010.
E. Dal Martello, S. Bernardis, G. Tranell, R. Larsen, and M. Di Sabatino: Powder Technol., 2012, vol. 224, pp. 209–16.
T. Hammouda and M. Pichavant: Eur. J. Min., 1999, vol. 11, pp. 637–53.
H. Deer and J. Zussman: An Introduction to The Rock-Forming Minerals. Longman Scientific and Technical, New York, 1992
R.B. Larsen, I. Henderson, P.M. Ihlen, F. Jacamon: Contrib. Min. Petrol, 2004, vol. 147, pp. 615–28.
I. Samson, A. Anderson, and D. Marshall: Fluid Inclusions: Analysis and Interpretation, vol. 32. Mineralogical Association of Canada, Vancouver, 2003.
B. Flem, R.B. Larsen, A. Grimstvedt, and J. Mansfeld: Chem. Geol., 2002, vol. 182, pp. 237–47.
G.H. Frischat: Ber Deut Keram Ges, 1970, vol. 47, pp. 364–68.
J. Verhoogen: Am Min., 1952, vol. 37, pp. 637–55.
G.H. Frischat: J. Am. Ceram. Soc., 1970, vol. 53, pp. 357–60.
R. Pankrath and O.W. Florke: Eur. J. Min., 1994, vol. 6, pp. 435–57.
S.C. Penniston-Dorland: Am Min., 2001, vol. 86 (5–6), pp. 652–66.
H.D. Stock and G. Lehmann: J. Phys. Chem. Solids, 1976, vol. 38, pp. 243–46.
D.J. Cherniak, E.B. Watson, and D.A. Wark: Chem. Geol., 2007, vol. 236, pp. 65–74.
A.C.D. Chaklader and A.L. Roberts: J. Am. Cer. Soc., 1961, vol. 44 (1), pp. 35–41.
V. Presser and K.G. Nickel: Crit. Rev. Solid State Mater. Sci., 2008, vol. 33, pp. 1–99.
A. Amington and J. Balascio: 38th Annual Symposium on Frequency Control, 1984, pp. 3–7.
A. Muller, W. B.J., and M. Smith, Mineralogical Magazine, 2005. 69(4): p. 381-401.
Larsen, R.B., F. Jacamon, and A. Kronz, Mineralogical Magazine, 2009. 73(4): p. 691-707.
R.B. Larsen, I. Henderson, and P.M. Ihlen: Contrib. Min. Petrol, 2004, vol. 147, pp. 615–28.
E.J.W. Whittaker and R. Muntus: Geochim Cosmochim Ac, 1970, vol. 34, pp. 945–56.
E. Dal Martello, G. Tranell, O. Ostrovski, G. Zhang, O. Raaness, R. Larsen, and P. Koshy: (2011). Metall. Mater. Trans. B 42(5): 939-950.
E. Dal Martello, G. Tranell, S. Gaal, and L. Arnberg: ISIJ Int., 2011. 51(9): p. 1492-1496.
Stoch, L., M. Laczka, and I. Waclawska, Mineralogia Polonica, 1985. 16(2): p. 43-54.
Astimex: http://astimex.com/com/catalog/min.html. 26th June 2011.
Australia Co.S.: Coal and Coke-Analysis and testing Part4: Coke -Proximate analysis. 2006, Standards Australia GPO Box 476, Sydney, NSW, Australia, 2001.
V. Andersen: Investigation of Thermal Properties of Quartz for the Silicon Industry Under Reducing Atmosphere, Department of materials science and engineering. NTNU, Trondheim, 2009.
T. Kawasaki and H. Ishizuka: J. Miner. Petrol. Sci., 2008, vol. 103, pp. 255–65.
Scherer, G., P.J. Vergano, and D.R. Uhlmann, Phys. Chem. Glasses, 1970. 11(3): p. 53-58.
Mitra, S., Trans. J. Brit. Ceram. Soc., 1977. 76(4): p. 71-74.
V. Balek, J. Fusek, J. Křiž, and M. Murat: Thermoc. Acta, 1995, vol. 262, pp. 209–14.
Benezet, J.C. and A. Benhassaine, Powder Technol 1999. 105(1) pp 167–71.
Steinike, U. and K. Tkáčová, J Mater Synth Proces, 2000. 8: p. 197-203.
Damn, C. and W. Peukert, Langmuir, 2009. 25: p. 2264-2270.
Ford, H.M., S.M. Auerbach, and P.A. Monson, J. Chem. Phys., 2004. 121(17): p. 8415-8422.
B. Zhu, Y. Sun, and C. Xie: J. China Coal Soc., 2008, pp. 1049–52.
J.B. Fein, J.J. Hemley, W.M. D'Angelo, A. Komninou, and D.A. Sverjensky: Geochim Cosmochim Ac., 1992, vol. 56, pp. 3179–90.
M. Gemeinert, M. Gaber, I. Hager, M. Willfahrt, and D. Bortschuloun: Neues Jahrbuch Miner. Abh., 1992. 165(1): p. 19-27.
P. Atkins and J. de Paula: Atkins’ Physical Chemistry, 7th ed. Oxford University Press Inc, New York, 2002.
Acknowledgments
The authors appreciate the inspiring discussions with Kristian Drivenes and Jeffery Kline; this study was possible thanks to the excellent technical help of John Sharp, Xing Xing, and Xiaohan Wan. Thanks are due also to Morten Raaneess and Syverin Lierhagen for their analyses. This study was partially funded by ELKEM’s research fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted: June 15, 2012.
Rights and permissions
About this article
Cite this article
Dal Martello, E., Tranell, G., Ostrovski, O. et al. Trace Elements in the Si Furnace. Part I: Behavior of Impurities in Quartz During Reduction. Metall Mater Trans B 44, 233–243 (2013). https://doi.org/10.1007/s11663-012-9717-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9717-4