Abstract
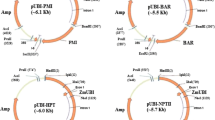
Agrobacterium-mediated transformation of Carrizo citrange [Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.] with a binary vector containing a novel bifunctional reporter–selection fusion gene comprising an in-frame fusion between the manA gene and egfp gene is detailed. This system combined the phosphomannose isomerase positive selection system with the ability to monitor gene expression in a non-destructive manner using EGFP. Transgenic plants stably expressing the EGFP protein were regenerated following Agrobacterium-mediated transformation using a vector containing this fusion gene. We also obtained comparable transformation efficiencies when Carrizo explants were transformed using another Agrobacterium strain containing a binary vector with a bifunctional egfp–nptII fusion gene. Regenerating shoots were selected on medium containing 15 g L−1 mannose supplemented with 5 g L−1 sucrose for the manA-based selection or on medium containing 100 mg L−1 kanamycin for the nptII-based selection. Our results indicated that the mannose-based antibiotic-free selection combined with visual identification of transgenic shoots using EGFP allows for early elimination of escape non-transgenic shoots and can provide a viable alternative to antibiotic-based selection systems in the genetic transformation of citrus and other crops.









Similar content being viewed by others
References
Ananthakrishnan G.; Orbovic V.; Pasquali G.; Calovic M.; Grosser J. W. Transfer of citrus tristeza virus (CTV)-derived resistance candidate sequences to four grapefruit cultivars through Agrobacterium-mediated genetic transformation. In Vitro Cell. Dev Biol Plant 43: 593–601; 2007.
Ballester A.; Cervera M.; Pena L. Evaluation of selection strategies alternative to nptII in genetic transformation of citrus. Plant Cell Rep 27: 1005–1015; 2008.
Boscariol R. L.; Almeida W. A. B.; Derbyshire M. T. V. C.; Mourao-Filho F. A. A.; Mendes B. M. J. The use of the PMI/mannose selection system to recover transgenic sweet orange plants (Citrus sinensis (L.) Osbeck). Plant Cell Rep 22: 122–128; 2003.
Brigneti G.; Voinnet O.; Li W. X.; Ji L. H.; Ding S. W.; Baulcombe D. C. Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J 17: 6739–6746; 1998.
Burrow M. D.; Chlan C. A.; Sen P.; Murai N. High frequency generation of transgenic tobacco plants after modified leaf disk co-cultivation with Agrobacterium tumefaciens. Plant Mol Biol Report 8: 124–139; 1990.
Cervera M.; Pina J. A.; Juarez J.; Navarro L.; Pena L. Agrobacterium mediated transformation of citrange: factors affecting transformation and regeneration. Plant Cell Rep 18: 271–278; 1998.
Cubitt A. B.; Heim R.; Adams S. R.; Boyd A. E.; Gross L. A.; Tsien R. Y. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci 20: 448–455; 1995.
Dale P. Spread of engineered genes to wild relatives. Plant Physiol 100: 13–15; 1992.
Dominguez A.; Cervera M.; Perez R. M.; Romero J.; Fagoaga C.; Cubero J.; Lopez M. M.; Juarez J. A.; Navarro L.; Pena L. Characterization of regenerants obtained under selective conditions after Agrobacterium-mediated transformation of citrus explants reveals production of silenced and chimeric plants at unexpected high frequencies. Mol Breed 14: 171–183; 2004.
Duan Y. X.; Liu X.; Fan J.; Li D.; Wu R. C.; Guo W. W. Multiple shoot induction from seedling epicotyls and transgenic citrus plant regeneration containing the green fluorescent protein gene. Bot Stud 48: 165–171; 2007.
Dutt M.; Grosser J. W. Evaluation of parameters affecting Agrobacterium-mediated transformation of Citrus. Plant Cell Tissue Organ Cult 98: 331–340; 2009.
Dutt M.; Li Z. T.; Dhekney S. A.; Gray D. J. Transgenic plants from shoot apical meristems of Vitis vinifera Thompson Seedless via Agrobacterium-mediated transformation. Plant Cell Rep 26: 2101–2110; 2007.
Ghorbel R.; Juarez J.; Navarro L.; Pena L. Green fluorescent protein as a valuable marker for efficient transformation and improved regeneration of recalcitrant woody plants. Theor Appl Genet 99: 350–358; 1999.
Goldsworthy A.; Street H. E. The carbohydrate nutrition of tomato roots VIII. The mechanism of the inhibition by d-mannose of the respiration of excised roots. Ann Bot 29: 45–58; 1965.
Hood E. E.; Gelvin S. B.; Melchers L. S.; Hoekema A. New Agrobacterium helper plasmids for gene transfer to plants. Trans Res 2: 208–218; 1993.
Johansen L. K.; Carrington J. C. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol 126: 930–938; 2001.
Joersbo M. Advances in the selection of transgenic plants using non-antibiotic marker genes. Physiol Plant 111: 269–272; 2001.
Joersbo M.; Donaldson I.; Kreiberg J.; Petersen S. G.; Brunstedt J.; Okkels F. T. Analysis of mannose selection used for transformation of sugar beet. Mol Breed 4: 111–117; 1998.
Joersbo M.; Mikkelsen J. D.; Brunstedt J. Relationship between promoter strength and transformation frequencies using mannose selection for the production of transgenic sugar beet. Mol Breed 6: 207–213; 2000.
Kobayashi S.; Uchimiya H. Expression and integration of a foreign gene in orange (Citrus sinensis Osb.) protoplasts by direct DNA transfer. Jpn J Genet 64: 91–97; 1989.
Kohler R. H. GFP for in vivo imaging of subcellular structures in plant cells. Trends Plant Sci 3: 317–320; 1998.
Kramer C.; Dimaio J.; Carswell G. K.; Shillito R. D. Selection of transformed protoplast-derived Zea mays colonies with phosphinothricin and a novel assay using the pH indicator chlorophenol red. Planta 190: 454–458; 1993.
Li D. D.; Shi W.; Deng X. X. Agrobacterium-mediated transformation of embryogenic calluses of Ponkan mandarin and the regeneration of plants containing the chimeric ribonuclease gene. Plant Cell Rep 21: 13–156; 2002.
Li Z.; Jayasankar S.; Gray D. J. Expression of a bifunctional green fluorescent protein (GFP) fusion marker under the control of three constitutive promoters and enhanced derivatives in transgenic grape (Vitis vinifera). Plant Sci 160: 877–887; 2001.
Lu R.; Folimonov A.; Shintaku M.; Li W. X.; Falk B. W.; Dawson W. O.; Ding S. W. Three distinct suppressors of RNA silencing encoded by a 20-kb viral RNA genome. Proc Natl Acad Sci USA 101: 15742–15747; 2004.
Lucca P.; Ye X. D.; Potrykus I. Effective selection and regeneration of transgenic rice plants with mannose as selective agent. Mol Breed 7: 43–49; 2001.
Mallory A. C.; Ely L.; Smith T. H.; Marathe R.; Anandalakshmi R.; Fagard M.; Vaucheret H.; Pruss G.; Bowman L.; Vance V. B. HC-Pro suppression of transgene silencing eliminates the small RNAs but not transgene methylation or the mobile signal. Plant Cell 13: 571–83; 2001.
Medina-Urrutia V.; Madera K. F. L.; Serrano P.; Ananthakrishnan G.; Grosser J. W.; Guo W. W. New intergeneric somatic hybrids combining Amblycarpa mandarin with six trifoliate/trifoliate hybrid selections for lime rootstock improvement. Hort Sci 39: 355–360; 2004.
Miles J. S.; Guest J. R. Nucleotide sequence and transcriptional start point of the phosphomannose isomerase gene (manA) of Escherichia coli. Gene 32: 41–48; 1984.
Mingeot-Leclercq M.; Glupczynski Y.; Tulkens P. M. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother 43: 727–737; 1999.
Molinari H. B. C.; Bespalhok J. C.; Kobayashi A. K.; Pereira L. F. P.; Vieira L. G. E. Agrobacterium tumefaciens-mediated transformation of Swingle citrumelo (Citrus paradisi Macf.× Poncirus trifoliata L. Raf.) using thin epicotyl sections. Sci Hortic 99: 379–385; 2004.
Moore G. A.; Jacono C. C.; Neiedigh J. L.; Lawrence S. D.; Cline K. Agrobacterium-mediated transformation of citrus stem segments and regeneration of transgenic plants. Plant Cell Rep 11: 238–242; 1992.
Mullins M. G.; Tang F. C.; Facciotti D. Agrobacterium mediated genetic transformation of grapevines: transgenic plants of Vitis rupestris Scheele and buds of Vitis vinifera. Bio/Technology 8: 1041–1045; 1990.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497; 1962.
Negrotto D.; Jolley M.; Beer S.; Wenck A. R.; Hansen G. The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via transformation. Plant Cell Rep 19: 798–803; 2000.
Pena L.; Cervera M.; Juarez J.; Ortega C.; Pina J. A.; Duran-Vila N.; Navarro L. High efficiency Agrobacterium-mediated transformation and regeneration of citrus. Plant Sci 104: 183–191; 1995.
Ramesh S.; Kaiser B. N.; Franks T. K.; Collins G. G.; Sedgley M. Improved methods in Agrobacterium-mediated transformation of almond using positive (mannose/pmi) or negative (kanamycin resistance) selection-based protocols. Plant Cell Rep 25: 821–828; 2006.
Reed J.; Privalle L.; Powell M. L.; Meghji M.; Dawson J.; Dunder E.; Suttie J.; Wenck A.; Launis K.; Kramer C.; Chang Y. F.; Hansen G.; Wright M. Phosphomannose isomerase: an efficient selectable marker for plant transformation. In Vitro Cell. Dev Biol Plant 37: 127–132; 2001.
Stewart J. C. N. The utility of green fluorescent protein in transgenic plants. Plant Cell Rep 20: 376–382; 2001.
Sheu Hwa C. S.; Lewis D. H.; Walker D. A. Stimulation of photosynthetic starch formation by sequestration of cytoplasmic orthophosphate. New Phytol 74: 383–392; 1975.
Voinnet O.; Rivas S.; Mestre P.; Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33: 949–956; 2003.
Zhang P.; Puonti-Kaerlas J. PEG-mediated cassava transformation using positive and negative selection. Plant Cell Rep 19: 1041–1048; 2000.
Zhu Y. J.; Agbayani R.; McCafferty H.; Albert H. H.; Moore P. H. Effective selection of transgenic papaya plants with the PMI/Man selection system. Plant Cell Rep 24: 426–432; 2005.
Acknowledgments
We thank Dr. Dennis J. Gray and Dr. Zhijian Li (MREC, University of Florida/IFAS, Apopka, FL, USA) for providing us with a construct containing the bifunctional egfp–nptII fusion gene. Gary Barthe and Monica Vasconcellos are thanked for their technical assistance. We also thank FCPRAC (Florida Citrus Production Research and Advisory Council) for grants supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Ranch
Rights and permissions
About this article
Cite this article
Dutt, M., Lee, D.H. & Grosser, J.W. Bifunctional selection–reporter systems for genetic transformation of citrus: mannose- and kanamycin-based systems. In Vitro Cell.Dev.Biol.-Plant 46, 467–476 (2010). https://doi.org/10.1007/s11627-010-9300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-010-9300-0