Abstract
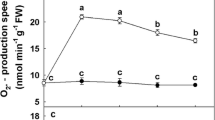
Hyperhydricity is a physiological disorder frequently affecting shoots propagated in vitro. Since it negatively affects shoot multiplication vigor, and impedes the successful transfer of micropropagated plants to in vivo conditions, hyperhydricity is a major problem in plant tissue culture. In commercial plant micropropagation, there are reports of up to 60% of cultured shoots or plantlets which demonstrate hyperhydricity, which reflects the pervasiveness of this problem. The phenomenon has been correlated to water availability, microelements, and/or hormonal imbalance in the tissue culture. In this study, the ultrastructure and the characteristics of reactive oxygen species between hyperhydric and normal shoots of garlic were studied. We observed that in some cells of hyperhydric tissues, the intranuclear inclusion was separated, the mitochondrion was swollen and its intracristae had splits, the organelles were compressed against the cell wall, and the chloroplasts and intergranal thylakoids were also compressed. Additionally, the content of chlorophyll and soluble protein in hyperhydric shoots decreased significantly. For instance, chlorophyll a decreased 43.61%, chlorophyll b decreased 49.29%, chlorophyll a+b decreased 48.10%, and soluble protein dropped 47.36%. In contrast, the O2 generation rate and H2O2 level increased 45.36% and 63.98%, respectively, obviously higher than the normal shoots. Lipoxygenase activity and malondialdehyde content in the hyperhydric shoots increased significantly, while the electrolyte leakage rose, indicating a serious membrane lipid peroxidatic reaction. Superoxide dismutase, peroxidase, catalase, glutathione peroxidase, and ascorbate peroxidase activities in hyperhydric tissue were all significantly higher than in normal leaf tissue. The antioxidant metabolism demostrated a close connection between hyperhydricity and reactivated oxygen species.





Similar content being viewed by others
References
Ahmed S.; Nawata E.; Hosokawa M.; Domae Y.; Sakuratani T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 163: 117–123; 2002. doi:10.1016/S0168-9452(02)00080-8.
Alir A.; Mayber A. Changes in activity of Malate Dehydrogenase, Catalase, Peroxidase and Superoxide Dismutase in leaves of Halimione Portulacoides L. exposed to high sodium chloride concentrations. Ann. Bot. 47: 75–85; 1981.
Asada K.; Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle D. J.; Osmond C. R.; Arntzen C. J. (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–286; 1987.
Ayabe M.; Sumi S. Establishment of a novel tissue culture method, stem-disc culture, and its practical application to micropropagation of garlic (Allium sativum L.). Plant Cell Rep. 17: 773–779; 1998. doi:10.1007/s002990050481.
Ayabe M.; Sumi S. A novel and efficient tissue culture method—“stem-disc dome culture”-for producing virus-free garlic (Allium sativum L.). Plant Cell Rep. 20: 503–507; 2001. doi:10.1007/s002990100358.
Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254; 1976. doi:10.1016/0003-2697(76)90527-3.
Caro A.; Puntarulo D. Effect of in vivo ion supplementation on oxygen radical production by soybean roots. Biochim. Biophys. Acta 1291: 245–251; 1996.
Debergh P. C.; Harbaoui Y.; Lemeur R. Mass propagation of globe artichoke (Cynara scolymus): evaluation of different hypotheses to overcome vitrification with special reference to water potential. Physiol. Plant. 53: 181–187; 1981. doi:10.1111/j.1399-3054.1981.tb04130.x.
Del Rio L. A.; Corpas F. J.; Sandalio L. M.; Palma J. M.; Barroso J. B. Plant peroxisomes, reactive oxygen metabolism and nitric oxide. IUBMB Life 55: 71–81; 2003. doi:10.1080/1521654031000094694.
Dhindsa R. S.; Plumb-Dhinsa P. L.; Thorpe T. A. Leaf senescence correlated with increased levels of membrane perme-ability and lipid peroxidation and decreased levels of super-oxide dismutase and colatase. Exp. Bat. 32: 93–101; 1981. doi:10.1093/jxb/32.1.93.
Droog F. Plant glutathione S-transferases, a tale of Theta and Tau. J. Plant Growth Reg. 16: 95–107; 1997. doi:10.1007/PL00006984.
Elstner E. F. Oxygen radicals—biochemical basis for their efficacy. Klin. Wochenschr. 69: 949–956; 1991. doi:10.1007/BF01645138.
Enrique O.; Abel P. The subcellular localization of peroxidase and the implication of oxidative stress in hyperhydrated leaves of regenerated carnation plants. Plant Sci. 130: 97–105; 1997. doi:10.1016/S0168-9452(97)00214-8.
Foyer C. H.; Lopez-Delgado H.; Dat J. F.; Scott I. M. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol. Plant. 100: 241–254; 1997. doi:10.1111/j.1399-3054.1997.tb04780.x.
Franck T.; Kevers C.; Gaspar T. Protective enzymatic systems against activated oxygen species compared in normal and vitrified shoots of Prunus avium L.L. raised in vitro. Plant Growth Regul. 16: 253–256; 1995. doi:10.1007/BF00024782.
Giannoplitis C. N.; Ries S. K. Superoxide dismutase: occur-rencein higher plants. Plant Physiol. 59: 309; 1977.
Gupta A. S.; Webb R. P.; Holaday A. S.; Allen D. Overexpression of SOD protects plants from oxidative stress. Induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants. Plant Physiol. 103: 1067–1073; 1993.
Hagege D. Habituation in plant cell cultures: adaptation to free radical attacks. Soc. Biol. 189: 1183–1190; 1995.
Hossain M. A.; Asada K. Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol. 25: 85–92; 1984.
Jagtap V.; Bhargava S. Variation in antioxidant metabolism of drought tolerant and drought susceptible varieties of Sorghum bicolor L. Moench, exposed to highlight, low water and high temperature stress. J. Plant Physiol. 145: 195–197; 1995.
Kang H. M.; Saltveit E. Effect of chilling on antioxidant enzymes and DPPH-radical scavenging activity of high- and low-vigour cucumber seedling radicles. Plant Cell Environ. 25: 1233–1238; 2002. doi:10.1046/j.1365-3040.2002.00915.x.
Kevers C.; Coumans M.; Coumans-Gilles F.; Gaspar T. Physiological and biochemical events leading to vitrification of shoots cultured in vitro. Physiol. Plant. 61: 69–74; 1984. doi:10.1111/j.1399-3054.1984.tb06102.x.
Kevers C.; Franck T.; Strasser R. J.; Dommes J.; Gaspar T. Hyperhydricity of micropropagated shoots: a typically stress-induced change of physiological state. Plant. Cell. Tiss. Org. Cult. 77: 181–191; 2004. doi:10.1023/B:TICU.0000016825.18930.e4.
Le D. F.; Huault C.; Gaspar T.; Billard J. P. Does altered nitrogen metabolism and H2O2 accumulation explain the vitrified status of the fully habituated callus of Beta ulgaris L. Plant Cell Tiss. Org. Cult. 35: 69–74; 1993.
Macpherson A. N.; Terlfer A.; Barber J. Biochim. Biophys. Acta 1143: 301–309; 1993. doi:10.1016/0005-2728(93)90201-P.
McKersie B. D.; Leshem Y. Y. Stress and stress coping in cultivated plants. Kluwer Academic Publishers, Dordrecht; 1994.
Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405–410; 2002. doi:10.1016/S1360-1385(02)02312-9.
Navari-Izzo J.; Quartacci M. F.; Sgherri C. L. M. Superoxide generation in relation to dehydration and rehydration. Biochem. Soc. Trans. 24: 447–451; 1996.
Olmos E.; Hellin E. Ultrastructural differences of hyperhydric and normal leaves from regenerated carnation plants. Sci. Hortic. (Amsterdam) 75: 91–101; 1998. doi:10.1016/S0304-4238(98)00096-X.
Patra J.; Panda K. K.; Panda B. B. Differential induction of adaptive responses by paraquat and hydrogen peroxide against the genotoxicity of methyl mercuric chloride, maleic hydrazide and ethyl methyl sulphonate in plant cells in vivo. Mutation Res. 393: 215–222; 1997.
Piqueras A.; Cortina M.; Serna M. D. Polyamines and hyperhydricity in micropropagated carnation plants. Plant Sci. 162: 671–678; 2002. doi:10.1016/S0168-9452(02)00007-9.
Piqueras A.; Han B. H.; Van J. M. Effect of different environmental conditions in vitro on sucrose metabolism and antioxidant enzymatic activities in cultured shoots of Nicotiana tabacum L. Plant Growth Regul. 25: 5–10; 1998. doi:10.1023/A:1005961632380.
Putter J. Peroxidases. In: Bergmeyer H. U. (ed) Methods of enzymatic analysis: II. Academic Press, New York, pp 685–690; 1974.
Reddy A. R.; Chaitanya K. V.; Jutur P. P.; Sumithra K. Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ. Exp. Bot. 52: 33–42; 2004. doi:10.1016/j.envexpbot.2004.01.002.
Sairam R. K.; Rao K. V.; Srivastava G. C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress. Plant Sci. 163: 1037–1046; 2002. doi:10.1016/S0168-9452(02)00278-9.
Shi L. B.; Li Y. Y. Effects of water stress on several physiological indexes and ultrastructure of chloroplast in winter wheat seedlings. Plant Physiology Communications 2: 28; 1990.
Shigeoka S.; Ishikawa T.; Tamoi M.; Miyagawa Y.; Takeda T.; Yabuta Y. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 53: 1305–1319; 2002. doi:10.1093/jexbot/53.372.1305.
Sies H. Oxidative stress. Amer. J. Med. 91: 31–38; 1991. doi:10.1016/0002-9343(91)90281-2.
Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 125: 27–58; 1993. doi:10.1111/j.1469-8137.1993.tb03863.x.
Stoeva N.; Berova M.; Zlatev Z. Effect of arsenic on some physiological indices in maize (Zea mays L.). J. Environ. Protec. Ecol. 4: 496–801; 2003.
Surrey K. Spectrophotometric methed for determination of lipoxidase activity. Plant Physiol. 39: 65–70; 1963.
Thierry F.; Claire K.; Thomas G.; Jacques D.; Doey C. Hyperhydricity of Prunus avium shoots cultured on gelrite: a controlled stress response. Plant Physiol. Biochem. 42: 519–527; 2004. doi:10.1016/j.plaphy.2004.05.003.
Uchida A.; Andre T. J.; Takashi H. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 163: 515–523; 2002. doi:10.1016/S0168-9452(02)00159-0.
Velikova V.; Yordanov I.; Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 151: 59–66; 2000. doi:10.1016/S0168-9452(99)00197-1.
Wang A. G.; Luo G. H. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol. Commun. 26: 55–57; 1990.
Wang J. F.; Li X. X.; Jia C. L. Preliminary study on the physiological characteristics and formation mechanism of the vitreous plantlets in Gypsophila elegans cultured in vitro (in Chinese). Plant J. 5: 74–77; 1997.
Wintermans J. F.; De Mots A. Spectrophotometric characteristics of chlorophyll a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 109: 448–453; 1965. doi:10.1016/0926-6585(65)90170-6.
Wiseman H.; Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 313: 17–29; 1996.
Yahraus T.; Chandra S.; Legendre L.; Low P. S. Evidence for a mechanically induced oxidative burst. Plant Physiol. 109: 1259–1266; 1995.
Zhao J.; Yang W. J. Relationships of oxygen free radicals stress and occurrence of vitrified-shoots during micro-propagation of Pyrus ‘pingbo No.1’ (pyrus pyrifoliacult var. pingbo No.1) in vitro (in Chinese). Acta. Bot. Boreal. Occident. Sin. 18(2): 183–189; 1998.
Zhao Y. G.; Xu J. X. Mitochondria, reactive oxygen species and apoptosis. Progress In Biochemistry and Biophysics 28(2): 168–171; 2001.
Zlatev Z. S.; Lidon F. C.; Ramalho J. C.; Yordanov I. T. Comparison of resistance to drought of three bean cultivars. Biol. Plant. 50(3): 389–394; 2006. doi:10.1007/s10535-006-0054-9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: E. Bunn
Rights and permissions
About this article
Cite this article
Wu, Z., Chen, L.J. & Long, Y.J. Analysis of ultrastructure and reactive oxygen species of hyperhydric garlic (Allium sativum L.) shoots. In Vitro Cell.Dev.Biol.-Plant 45, 483–490 (2009). https://doi.org/10.1007/s11627-008-9180-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-008-9180-8