Abstract
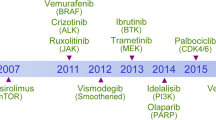
The emergence of molecularly targeted agents in oncology has not only revolutionized the care of cancer patients, but also changed the daily practice of medical oncologists. Molecularly targeted agents indeed often differ from traditional cytotoxic agents by their administration schedules and routes, their toxicity profiles, and/or the assessment of their antitumor activity. In addition, the observation that molecularly targeted agents sometimes have limited antitumor activity as single agents has led clinical investigators to combine molecularly targeted agents together or with cytotoxic agents. We review here the current challenges for the early clinical development of anticancer agents in the era of molecularly targeted agents. We focus on the choice of end points in phase I oncology clinical trials, as well as on the choice of dose escalation methods with an emphasis on available dose escalation methods for molecularly targeted agents and for combination trials.
Similar content being viewed by others
References
DiMasi JA, Grabowski HG (2007) Economics of new oncology drug development. J Clin Oncol 25:209–216
Von Hoff DD (1998) There are no bad anticancer agents, only bad clinical trial designs—twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res 4:1079–1086
Ranson M, Hammond LA, Ferry D et al (2002) ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol 20:2240–2250
Thatcher N, Chang A, Parikh P et al (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366:1527–1537
Shepherd FA, Rodrigues Pereira J et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–139
Paez JG, Jänne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Pao W, Miller V, Zakowski M et al (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101:13306–13311
Henderson IC, Berry DA, Demetri GD et al (2003) Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976–983
Fisher B, Anderson S, Wickerham DL et al (1997) Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol 15:1858–1869
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25:3362–3371
Strevel EL, Siu LL (2009) Cardiovascular toxicity of molecularly targeted agents. Eur J Cancer 45(Suppl 1):318–331
Asnacios A, Naveau S, Perlemuter G (2009) Gastrointestinal toxicities of novel agents in cancer therapy. Eur J Cancer 45(Suppl 1):332–342
Segaert S, Chiritescu G, Lemmens L et al (2009) Skin toxicities of targeted therapies. Eur J Cancer 45(Suppl 1):295–308
Carles J, Morales R, Perez JM et al (2009) Management and interpretation of novel toxicities of molecular targeted therapies: renal toxicities. Eur J Cancer 45(Suppl 1):309–317
Roberts TG Jr, Goulart BH, Squitieri L et al (2004) Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA 292:2130–2140
Horstmann E, McCabe MS, Grochow L et al (2005) Risks and benefits of phase I oncology trials, 1991 through 2002. N Engl J Med 352:895–904
Postel-Vinay S, Arkenau HT, Olmos D et al (2009) Clinical benefit in Phase-I trials of novel molecularly targeted agents: does dose matter? Br J Cancer 100:1373–1378
Le Tourneau C, Vidal L, Siu LL (2008) Progress and challenges in the identification of biomarkers for EGFR and VEGFR targeting anticancer agents. Drug Resist Updat 11:99–109
Ratain MJ, Glassman RH (2007) Biomarkers in phase I oncology trials: signal, noise, or expensive distraction? Clin Cancer Res 13:6545–6548
Druker BJ, Talpaz M, Resta DJ et al (2001) Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1031–1037
Atkins MB, Hidalgo M, Stadler WM et al (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22:909–918
Kabbinavar F, Hurwitz HI, Fehrenbacher L et al (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Sleijfer S, Wiemer E (2008) Dose selection in phase I studies: why we should always go for the top. J Clin Oncol 26:1576–1578
Goulart BH, Clark JW, Pien HH et al (2007) Trends in the use and role of biomarkers in phase I oncology trials. Clin Cancer Res 13:6719–6726
Sauter G, Lee J, Bartlett JM et al (2009) Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 27:1323–1333
Allegra CJ, Jessup JM, Somerfield MR et al (2009) American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27:2091–2096
Mandrekar SJ, Sargent DJ (2009) Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J Clin Oncol 27:4027–4034
Parulekar WR, Eisenhauer EA (2004) Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst 96:990–997
Le Tourneau C, Lee JJ, Siu LL (2009) Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 101:708–720
Gordon MS, Margolin K, Talpaz M et al (2001) Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19:843–850
Takimoto CH (2009) Pharmacokinetics and pharmacodynamic biomarkers in early oncology drug development. Eur J Cancer 45(Suppl 1):436–438
Adjei AA (2006) What is the right dose? The elusive optimal biologic dose in phase I clinical trials. J Clin Oncol 24:4054–4055
Booth CM, Calvert AH, Giaccone G et al (2008) Endpoints and other considerations in phase I studies of targeted anticancer therapy: recommendations from the task force on Methodology for the Development of Innovative Cancer Therapies (MDICT). Eur J Cancer 44:19–24
Graham MA, Workman P (1992) The impact of pharmacokinetically guided dose escalation strategies in phase I clinical trials: critical evaluation and recommendations for future studies. Ann Oncol 3:339–347
Agrawal M, Grady C, Fairclough DL et al (2006) Patients’ decision-making process regarding participation in phase I oncology research. J Clin Oncol 24:4479–4484
Nurgat ZA, Craig W, Campbell NC et al (2005) Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br J Cancer 92:1001–1005
Daugherty C, Ratain MJ, Grochowski E et al (1995) Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 13:1062–1072
Hamberg P, Verweij J (2009) Phase I drug combination trial design: walking the Ttightrope. J Clin Oncol 27:4441–4443
Haddad RI, Tishler RB, Norris C et al (2009) Phase I study of C-TPF in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol 27:4448–4453
Arkenau HT, Olmos D, Ang JE et al (2008) Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer 98:1029–1033
Italiano A, Massard C, Bahleda R et al (2008) Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Ann Oncol 19:787–792
Fong PC, Boss DS, Yap TA et al (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123–134
Von Hoff DD, LoRusso PM, Rudin CM et al (2009) Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 361:1164–1172
Dong M, Ning Z, Newman MJ et al (2009) Phase I study of chidamide (CS055/HBI-8000), a novel histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. J Clin Oncol 27:15s, abstract 3529
Dent SF, Eisenhauer EA (1996) Phase I trial design: are new methodologies being put into practice? Ann Oncol 7:561–566
Eisenhauer EA, O’Dwyer PJ, Christian M et al (2000) Phase I clinical trial design in cancer drug development. J Clin Oncol 18:684–692
Rogatko A, Schoeneck D, Jonas W et al (2007) Translation of innovative designs into phase I trials. J Clin Oncol 25:4982–4986
Simon R, Freidlin B, Rubinstein L et al (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89:1138–1147
Collins JM, Grieshaber CK, Chabner BA (1990) Pharmacologically guided phase I clinical trials based upon preclinical drug development. J Natl Cancer Inst 82:1321–1326
O’Quigley J, Pepe M, Fisher L (1990) Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 46:33–48
Babb J, Rogatko A, Zacks S (1998) Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med 17:1103–1120
Friedman HS, Kokkinakis DM, Pluda J et al (1998) Phase I trial of O6-benzylguanine for patients undergoing surgery for malignant glioma. J Clin Oncol 16:3570–3575
Hunsberger S, Rubinstein LV, Dancey J et al (2005) Dose escalation trial designs based on a molecularly targeted endpoint. Stat Med 24:2171–2181
Polley MY, Cheung YK (2008) Two-stage designs for dose-finding trials with a biologic endpoint using stepwise tests. Biometrics 64:232–241
Zhang W, Sargent DJ, Mandrekar S (2006) An adaptive dose-finding design incorporating both toxicity and efficacy. Stat Med 25:2365–2383
Thall PF, Cook JD (2004) Dose-finding based on efficacy-toxicity trade-offs. Biometrics 60:684–693
Mandrekar SJ, Cui Y, Sargent DJ (2007) An adaptive phase I design for identifying a biologically optimal dose for dual agent drug combinations. Stat Med 26:2317–2330
Yin G, Li Y, Ji Y (2006) Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics 62:777–787
Thall PF, Millikan RE, Mueller P et al (2003) Dose-finding with two agents in Phase I oncology trials. Biometrics 59:487–496
Huang X, Biswas S, Oki Y et al (2007) A parallel phase I/II clinical trial design for combination therapies. Biometrics 63:429–436
Yuan Y, Yin G (2008) Sequential continual reassessment method for two-dimensional dose finding. Stat Med 27:5664–5678
Yin G, Yuan YA (2009) Latent contingency table approach to dose finding for combinations of two agents. Biometrics 65:866–875
Houede N, Thall PF, Nguyen H et al (2009) Utility-based optimization of combination therapy using ordinal toxicity and efficacy in phase I/II trials. Biometrics. doi:10.1111/j.1541-0420.2009.01302.x
Cannistra SA (2008) Challenges and pitfalls of combining targeted agents in phase I studies. J Clin Oncol 26:3665–3667
Conflict of interest statement
No funds or benefits in any form have been received by the authors in support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le Tourneau, C., Diéras, V., Tresca, P. et al. Current challenges for the early clinical development of anticancer drugs in the era of molecularly targeted agents. Targ Oncol 5, 65–72 (2010). https://doi.org/10.1007/s11523-010-0137-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-010-0137-6