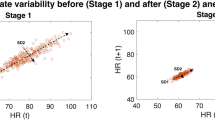
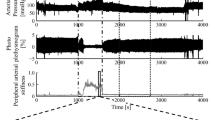
Abstract
This paper proposes a new method of evaluating autonomic nervous activity using the mechanical impedance of arterial walls and heart rate variability. The cardiovascular system is indispensable to life maintenance functions, and homeostasis is maintained by the autonomic nervous system. Accordingly, it is very important to be able to make diagnosis based on autonomic nervous activity within the body’s circulation. The proposed method was evaluated in surgical operations; the mechanical impedance of the arterial wall was estimated from arterial blood pressure and a photoplethysmogram, and heart rate variability was estimated using electrocardiogram R–R interval spectral analysis. In this paper, we monitored autonomic nervous system activity using the proposed system during endoscopic transthoracic sympathetic block surgery in eight patients with hyperhidrosis. The experimental results indicated that the proposed system can be used to estimate autonomic nervous activity in response to events during operations.






Similar content being viewed by others
References
Akselrod S, Gordon D, Ubel FA et al (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 10:220–222
Armentano RL, Simon A, Levenson J et al (1991) Mechanical pressure versus intrinsic effects of hypertension on large arteries in humans. Hypertension 18(5):657–664
Assalia A, Bahouth H, Ilivitzki A et al (2007) Thoracoscopic sympathectomy for primary palmar hyperhidrosis: resection versus transection—a prospective trial. World J Surg 31:1976–1979. doi:10.1007/s00268-007-9160-x
Bank AJ, Wilson RF, Kubo SH et al (1995) Direct effects of smooth muscle relaxation and contraction on in vivo human brachial artery elastic properties. Circ Res 77:1008–1016
Barra JG, Armentano RL, Levenson J et al (1993) Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res 73:1040–1050
Bootsma M, Swenne CA, Van Bolhuis HH et al (1994) Heart rate and heart rate variability as indexes of sympathovagal balance. Am J Physiol 266:1565–1571
Cerutti S, Goldberger AL, Yamamoto Y (2006) Recent advances in heart rate variability signal processing and interpretation. IEEE Trans Biomed Eng 53(1):1–3. doi:10.1109/TBME.2005.859777
Drott C, Gothberg G, Claes G (1995) Endoscopic transthoracic sympathectomy: an efficient and safe method for the treatment of hyperhidrosis. J Am Acad Dermatol 33(1):78–81
Fujiwara Y, Kurokawa S, Asakura Y et al (2007) Correlation between heart rate variability and haemodynamic fluctuation during induction of general anaesthesia: comparison between linear and non-linear analysis. Anaesthesia 62:117–121. doi:10.1111/j.1365-2044.2006.04933.x
Greenfield JC, Patel DJ (1962) Relation between pressure and diameter in the ascending aorta of man. Circ Res 10:778–781
Haney MF, Wiklund U (2007) Can heart rate variability become a screening tool for anesthesia-related hypotension? Acta Anaesthesiol Scand 51(10):1289–1291. doi:10.1111/j.1399-6576.2007.01517.x
Hanss R, Bein B, Weseloh H et al (2006) Heart rate variability predicts severe hypotension after spinal anesthesia. Anesthesiology 104:537–545
Hidaka S, Kawamoto M, Kurita S, Yuge O (2005) Comparison of the effects of propofol and midazolam on the cardiovascular autonomic nervous system during combined spinal and epidural anesthesia. J Clin Anesth 17(1):36–43. doi:10.1016/j.jclinane.2004.03.012
HRV (1996) Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology: Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065
Huang HH, Lee YH, Chan HL et al (2008) Using a short-term parameter of heart rate variability to distinguish awake from isoflurane anesthetic states. Med Biol Eng Comput 46(10):977–984. doi:10.1007/s11517-008-0342-y
Ihlen EA (2009) A comparison of two Hilbert spectral analyses of heart rate variability. Med Biol Eng Comput 47(10):1035–1044. doi:10.1007/s11517-009-0500-x
Kanaya N, Hirata N, Kurosawa S, Nakayama M, Namiki A (2003) Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology 98:34–40
Katayama K, Shimoda M, Maeda J, Takemiya T (1998) Endurance Exercise training increases peripheral vascular response in human fingers. Jpn J Physiol 48(5):365–371
Kato R, Sato J, Iuchi T, Higuchi Y (1999) Quantitative determination of arterial wall mechanics with pulse oximetric finger plethysmography. J Anesthesia 13:197–204
Knuttgen D, Trojan S, Weber M et al (2005) Pre-operative measurement of heart rate variability in diabetics: a method to estimate blood pressure stability during anaesthesia induction. Anaesthesist 54:442–449. doi:10.1007/s00101-005-0837-y
Kumazawa T, Yugi O, Furuya H (2002) Standard anesthesia. Igaku-Shoin Press, Tokyo, pp 220–231 (in Japanease)
Malliani A, Pagani M, Lombardi F et al (1991) Cardiovascular neural regulation explored in the frequency domain. Circulation 84:482–492
Nichols WW, O’Rourke MF (1998) McDonald’s blood flow in arteries: theoretical experimental and clinical principles, 4th edn. Arnold, London
Ohtomo N, Tanaka Y (1994) New method of time series analysis and ‘MemCalc’. A recent advance in time series analysis by maximum entropy method. Hokkaido University Press, Sapporo, pp 11–29
Parati G, Mancia G, Rienzo MD et al (2006) Point-counterpoint: cardiovascular variability is/is not an index of autonomic control of the circulation. J Appl Physiol 101(2):676–682. doi:10.1152/japplphysiol.00446.2006
Rajendra AU, Paul JK, Kannathal N et al (2006) Heart rate variability: a review. Med Biol Eng Comput 44(12):1031–1051. doi:10.1007/s11517-006-0119-0
Saeki N, Kawamoto M, Yuge O (2001) Quantitative view of peripheral circulation. In: 30th international educational and scientific symposium of the society of critical care medicine. San Francisco, USA
Sakane A, Tsuji T, Tanaka Y, Saeki N, Kawamoto M (2003) Estimating arterial wall impedance using a plethysmogram. In: Proceedings of the 29th annual conference of the IEEE industrial electronics society (IECON 03), vol 1. pp 580–585. doi:10.1109/IECON.2003.1280044
Sakane A, Tsuji T, Saeki N, Kawamoto M (2004) Discrimination of vascular conditions using a probabilistic neural network. J Robotics Mechatron 16(2):138–145
Sawada Y, Ohtomo N, Tanaka Y et al (1997) New technique for time series analysis combining the maximum entropy method and non-linear least squares method: its value in heart rate variability analysis. Med Biol Eng Comput 35:318–322
Suzuki J, Maeda J, Takemiya T (1994) Analysis of microvascular responses in the finger to changes in arm position during cold water stimulation. Jpn J Physiol 44(2):181–191
Tan BH, Shimizu H, Hiromoto K et al (2003) Wavelet transform analysis of heart rate variability to assess the autonomic changes associated with spontaneous coronary spasm of variant angina. J Electrocardiol 36(2):117–124. doi:10.1054/jelc.2003.50022
Taylor JA, Eckberg DL (1996) Fundamental relations between short-term R-R interval and arterial pressure oscillations in humans. Circulation 93:1527–1532
Tsuji T, Morasso PG, Goto K, Ito K (1995) Human hand impedance characteristics during maintained posture. Biol Cybern 72(6):475–485
Win NN, Fukayama H, Kohase H, Umino M (2005) The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability. Anesth Analg 101:97–102. doi:10.213/01.ANE.0000156204.89879.5C
Yamamoto Y, Hughson RL, Peterson JC (1991) Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol 71:1136–1142
Zhang R, Iwasaki K, Zuckerman JH et al (2002) Mechanism of blood pressure and R-R variability insights from ganglion blockade in humans. J Physiol 543(1):337–348
Acknowledgements
This work was supported by the Regional Innovation Creating System Enterprise for Ministry of Economy, Trade and Industry (RIETI) of Japan.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
In the program of MemCalc, a time series is assumed to be composed of underlying variation and fluctuating parts; the underlying variation is expressed as the function x uv (t), which can be given by a linear combination of sine and cosine functions
where f n is the frequency of the nth component, a n and b n are the amplitudes of the nth periodic component, Np is the total number of components, and a 0 is a constant that indicates the mean value of the time series. The value of f n is determined by the peaks in the power spectral density. Its estimate, P(f), can be expressed as
where P m is the output power of the prediction error filter of the order m, and γ m,k is the corresponding filter coefficient, m = 0, 1, 2,…, M (M = optimum filter order). P m and γ m,k are determined by Yule–Walker equations using Burg’s algorithm. Ohtomo and Tanaka [24] demonstrated that Eq. 5 gives a basis for determining the filter order, and the optimum order should be determined by the condition; the filter order is >1/f min, where f min is the minimum amongst the central frequencies of the components. In this program, the filter order was much higher than those obtained from the first minima of conventional information criteria. MemCalc does not cause distortion of the power calculation even if the underlying variation is changed.
Rights and permissions
About this article
Cite this article
Kutluk, A., Tsuji, T., Ukawa, T. et al. A novel online method to monitor autonomic nervous activity based on arterial wall impedance and heart rate variability. Med Biol Eng Comput 48, 351–359 (2010). https://doi.org/10.1007/s11517-010-0580-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-010-0580-7