Summary
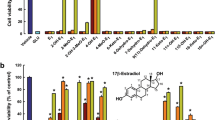
It is known that estrogen can protect neurons from excitotoxicity. Since isoflavones possess estrogen-like activity, it is of interest to determine whether isoflavones can also protect neurons from glutamate-induced neuronal injury. Morphological observation and lactate dehydrogenase (LDH) release assay were used to estimate the cellular damage. It is surprising that, contrary to estrogen, isoflavones, specifically genistein and daidzein, are toxic to primary neuronal culture at high concentration. Treatment of neurons with 50 μM genistein and daidzein for 24 h increased LDH release by 90% and 67%, respectively, indicating a significant cellular damage. Under the same conditions, estrogen such as 17β-estradiol did not show any effect on primary culture of brain cells. At 100 μM, both genistein and daidzein increased LDH release by 2.6- and 3-fold, respectively with a 30-min incubation. Furthermore, both genistein and daidzein at 50 μM increased the intracellular calcium level, [Ca2+]i, significantly. To determine their mode of action, genistein and daidzein were tested on glutamate and GABAA receptor binding. Both genistein and daidzein were found to have little effect on glutamate receptor binding, while the binding of [3H]muscimol to GABAA receptors was markedly inhibited. However, 17β-estradiol did not affect GABAA receptor binding suggesting that the toxic effect of genistein and daidzein could be due to their inhibition of the GABAA receptor resulting in further enhancement of excitation by glutamate and leading to cellular damage.
Similar content being viewed by others
References
Soghomonian J.J., Laprade N., Glutamate decarboxylase (GAD67 and GAD65) gene expression is increased in a subpopulation of neurons in the putamen of Parkinsonian monkeys. Synapse 27: 122–132, 1997
Wu J.Y., Bird E.D., Chen M.S., Huang W.M., Abnormalities of neurotransmitter enzymes in Huntington’s chorea. Neurochem. Res. 4: 575–586, 1979
Marczynski T.J., GABAergic deafferentation hypothesis of brain aging and Alzheimer’s disease revisited. Brain Res. Bull. 45: 341–379, 1998
Kash S.F., Johnson R.S., Tecott L.H., Noebels J.L., Mayfield R.D., Hanahan D., Baekkeskov S., Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA. 94: 14060–14065, 1997
Brinton R.D., Estrogens and Alzheimer’s disease. In: Marwah J. and Teitelbaum H. (Eds), Advances in Neurodegenerative Disorders, Vol. 2. Prominent Press, 1998, pp. 99–130
Sherwin B.B., Oestrogen and cognitive function throughout the female lifespan. Novartis Found Symp. 230: 188–196; discussion 196–201, 2000
Birg S.J., Is there a role for estrogen replacement therapy in the prevention and treatment of dementia? J. Am. Geriatr. Soc. 44: 878–880, 1996
Henderson V.W., Hormone Therapy and the Brain: A Clinical Perspective on the Role of Estrogen, 2000, Parthenon Publishing, New York
Mooradain A.D., Antioxidant properties of steroids. J. Steriod Biochem. Mol. Biol. 45: 509–511, 1993
Sugioka K., Shimosegawa Y., Nakano M., Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 210: 37–39, 1987
Akiyama T., Ogawara H., Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 201: 362–370, 1991
Constaninou A., Huberman E., Genistein as an inducer of tumor cell differentiation: possible mechanisms of action. Proc. Soc. Exp. Biol. Med. 208: 109–115, 1995
Kim H., Peterson T.G., Barnes S., Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am. J. Clin. Nutr. 68: 1418S–1425S, 1998
Brzezinski A., Debi A., Phytoestrigens: the “natural” selective estrogen receptor modulators? Eur. J. Obstet. Gynecol. Reprod. Biol. 85: 47–51, 1999
Osborne C.K., Zhao H., Fuqua S.A., Selective estrogen receptor modulators: structure, function, and clinical use. J. Clin. Oncol. 18: 3172–3186, 2000
An J., Tzagarakis-Foster C., Scharschmidt T.C., Lomri N., Leitman D.C., Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J. Biol. Chem. 276: 17808–17814, 2001
Pagliacci M.C., Smacchia M., Migliorati G., Grignani F., Riccardi C., Nicoletti I., Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur. J. Cancer. 30A: 1675–1682, 1994
Dubal D.B., Shughrue P.J., Wilson M.E., Merchenthaler I., Wise P.M., Kindy M.S., Wise P.M., Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 19: 6385–6393, 1999
Dubal D.B., Zhu H., Yu J., Rau S.W., Shughrue P.J., Merchenthaler I., Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. USA. 98: 1952–1957, 2001
Lee Y.H., Deupree D.L., Chen S.C., Kao L.S., Wu J.Y., Role of Ca2+ in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-mediated polyphosphoinositide turnover in primary neuronal cultures. J. Neurochem. 62: 2325–2332, 1994
Koh J.Y., Choi D.W., Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J. Neurosci. Methods 20: 83–90, 1987
Chen W.Q., Jin H., Nguyen M., Carr J., Lee Y.J., Hsu C.C., Faiman M.D., Schloss J.V., Wu J.Y., Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J. Neurosci. Res. 66: 612–619, 2001
Liao C.C., Lin H.S., Liu J.Y., Hibbard L.S., Wu J.Y., Purification and characterization of a benzodiazepine-like substance from mammalian brain. Neurochem. Res. 14: 345–352, 1989
Martin P.M., Horwitz K.B., Ryan D.S., McGuire W.L., Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 103: 1860–1867, 1978
Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y., Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262: 5592–5595, 1987
Markovits J., Linassier C., Fosse P., Coouprie J., Pierre J., Jacquemin-Sablon A., Saucier J.M., Le Pecq J.B., Larsen A.K., Inhibition effects of the tyrosine kinase inhibitor genistein n mammalian DNA topoisomerase II, Cancer Res. 49: 5111–5117, 1989
Pagliacci M.C., Smacchia M., Migliorati G., Grignani F., Riccardi C., Nicoletti I., Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur. J. Cancer. 30A: 1675–1682, 1994
Huang R.Q., Fang M.J., Dillon G.H., The tyrosine kinase inhibitor genistein directly inhibits GABAA receptors. Brain Res. Mol. Brain Res. 67: 177–183, 1999
Wu H., Jin Y., Wei J., Jin H., Sha D., Wu J.Y., Mode of action of taurine as a neuroprotector. Brain Res. 1038: 123–131, 2005
Wang C., Davis N., Colvin R.A. Genistein inhibits Na+/Ca2+ exchange activity in primary rat cortical neuron culture. Biochem. Biophys. Res. Commun. 223: 86–90, 1997
Linford N.J., Yang Y., Cook D.G., Dors D.M., Neuronal apoptosis resulting from high doses of the isoflavone genistein: role for calcium and P42/44 mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 299: 67–75, 2001
Acknowledgements
This work was supported in part by National Institutes of Health Grant NS37851, National Science Foundation Grant IBN-9723079, and the Schmidt Family Foundation at Florida Atlantic University. We would like to thank Wanda Dominger for excellent assistance in editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ying Jin, Heng Wu contributed equally to this article.
Rights and permissions
About this article
Cite this article
Jin, Y., Wu, H., Cohen, E.M. et al. Genistein and daidzein induce neurotoxicity at high concentrations in primary rat neuronal cultures. J Biomed Sci 14, 275–284 (2007). https://doi.org/10.1007/s11373-006-9142-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11373-006-9142-2