Abstract
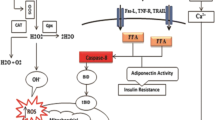
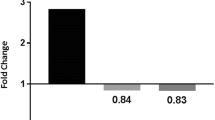
Chronic exposure to inorganic arsenic (iAs) or a high-fat diet (HFD) can produce liver injury. However, effects of HFD on risk assessment of iAs in drinking water are unclear. In this study, we examined how HFD and iAs interact to alter iAs-induced liver injury in C57BL/6 mice. Mice fed low-fat diet (LFD) or HFD were exposed to 3 mg/L iAs or deionized water for 10 weeks. Results showed that HFD changed intake and excretion of iAs by mice. Then, HFD increased the amount of iAs-induced hepatic DNA damage and amplified changes in pathways related to cell death and growth, signal transduction, lipid metabolism, and insulin signaling. Compared to gene expression profiles caused by iAs alone or HFD alone, insulin signaling pathway might play important roles in the interactive effects of iAs and HFD. Our data suggest that HFD increases sensitivity of mice to iAs in drinking water, resulting in increased hepatotoxicity. This study highlight that HFD might enhance the risk of iAs hepatotoxicity in iAs-polluted regions. The diet should be considered during risk assessment of iAs in drinking water.




Similar content being viewed by others
References
Ahmed MK et al (2011) Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere 84:143–149. doi:10.1016/j.chemosphere.2011.02.025
Alava P, Tack F, Laing GD, de Wiele TV (2012) HPLC-ICP-MS method development to monitor arsenic speciation changes by human gut microbiota. Biomed Chromatogr 26:524–533. doi:10.1002/bmc.1700
Altenburger R, Scholz S, Schmitt-Jansen M, Busch W, Escher BI (2012) Mixture toxicity revisited from a toxicogenomic perspective. Environ Sci Technol 46:2508–2522. doi:10.1021/es2038036
Arteel GE et al (2008) Subhepatotoxic exposure to arsenic enhances lipopolysaccharide-induced liver injury in mice. Toxicol Appl Pharmacol 226:128–139. doi:10.1016/j.taap.2007.08.020
Beyaz S et al (2016) High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531:53-+. doi:10.1038/nature17173
Brown KG, Ross GL (2002) Arsenic, drinking water, and health: a position paper of the American council on science and health. Regul Toxicol Pharmacol 36:162–174. doi:10.1006/rtph.2002.1573
Bundschuh J et al (2012) One century of arsenic exposure in Latin America: a review of history and occurrence from 14 countries. Sci Total Environ 429:2–35. doi:10.1016/j.scitotenv.2011.06.024
Collins AR, Ma AG, Duthie SJ (1995) The kinetics of repair of oxidative DNA-damage (strand breaks and oxidized pyrimidines) in human-cells. Mutat Res-DNA Repair 336:69–77
Davis AP, Murphy CG, Rosenstein MC, Wiegers TC, Mattingly CJ (2008) The comparative Toxicogenomics database facilitates identification and understanding of chemical-gene-disease associations: arsenic as a case study. BMC Med Genet 1:48. doi:10.1186/1755-8794-1-48
De Vizcaya-Ruiz A, Barbier O, Ruiz-Ramos R, Cebrian ME (2009) Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat Res Genet Toxicol Environ Mutagen 674:85–92. doi:10.1016/j.mrgentox.2008.09.020
Del Razo LM et al (2011) Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ Health 10:73. doi:10.1186/1476-069x-10-73
Ditzel EJ, Nguyen T, Parker P, Camenisch TD (2016) Effects of arsenite exposure during fetal development on energy metabolism and susceptibility to diet-induced fatty liver disease in male mice. Environ Health Perspect 124:201–209. doi:10.1289/ehp.1409501
Drobna Z, Walton FS, Paul DS, Xing WB, Thomas DJ, Styblo M (2010) Metabolism of arsenic in human liver: the role of membrane transporters. Arch Toxicol 84:3–16. doi:10.1007/s00204-009-0499-7
El-Hachem N et al (2016) Characterization of conserved toxicogenomic responses in chemically exposed hepatocytes across species and platforms. Environ Health Perspect 124:313–320. doi:10.1289/ehp.1409157
Franko A et al (2014) Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J Hepatol 60:816–823. doi:10.1016/j.jhep.2013.11.020
Frost FJ, Muller T, Petersen HV, Thomson B, Tollestrup K (2003) Identifying US populations for the study of health effects related to drinking water arsenic. J Expo Anal Environ Epidemiol 13:231–239. doi:10.1038/sj.jea.7500275
Huang CF et al (2015) Arsenic Exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect 123:1138–1144. doi:10.1289/ehp.1408663
Islam MR et al (2012) Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health 11. doi:10.1186/1476-069x-11-38
Jung UJ, Cho YY, Choi MS (2016) Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice Nutrients 8. doi:10.3390/nu8050305
Liu S, Guo X, Zhang X, Cui Y, Zhang Y, Wu B (2013) Impact of iron precipitant on toxicity of arsenic in water: a combined in vivo and in vitro study. Environ Sci Technol 47:3432–3438
Massey VL et al (2015) Oligofructose protects against arsenic-induced liver injury in a model of environment/obesity interaction. Toxicol Appl Pharm 284:304–314. doi:10.1016/j.taap.2015.02.022
Mazumder DN (2005) Effect of chronic intake of arsenic-contaminated water on liver. Toxicol Appl Pharmacol 206:169–175. doi:10.1016/j.taap.2004.08.025
Meliker JR, Wahl RL, Cameron LL, Nriagu JO (2007) Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health 6:4. doi:10.1186/1476-069x-6-4
Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS (2003) Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289:76–79
Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E (2006) Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiologic evidence. Environ Health Perspect 114:641–648. doi:10.1289/ehp.8551
Noeman SA, Hamooda HE, Baalash AA (2011) Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr 3:17. doi:10.1186/1758-5996-3-17
Paul DS, Hernandez-Zavala A, Walton FS, Adair BM, Dedina J, Matousek T, Styblo M (2007) Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol 222:305–314. doi:10.1016/j.taap.2007.01.010
Paul DS, Walton FS, Saunders RJ, Styblo M (2011) Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 119:1104–1109. doi:10.1289/ehp.1003324
Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Aposhian HV (2000) Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163:203–207. doi:10.1006/taap.1999.8872
Sasaki YF et al (2002) The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res 519:103–119
Schuhmacher-Wolz U, Dieter HH, Klein D, Schneider K (2009) Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects. Crit Rev Toxicol 39:271–298. doi:10.1080/10408440802291505
Smith AH, Steinmaus CM (2009) Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health 30:107–122. doi:10.1146/annurev.publhealth.031308.100143
Snedeker SM, Hay AG (2012) Do interactions between gut ecology and environmental chemicals contribute to obesity and diabetes? Environ Health Perspect 120:332–339
Tan M, Schmidt RH, Beier JI, Watson WH, Zhong H, States JC, Arteel GE (2011) Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol 257:356–364. doi:10.1016/j.taap.2011.09.019
Tseng CH et al (2000) Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect 108:847–851. doi:10.1289/ehp.00108847
Wiele TVD et al (2010) Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils. Environ Health Perspect 118:1004–1009
Wu J, Liu J, Waalkes MP, Cheng ML, Li L, Li CX, Yang Q (2008) High dietary fat exacerbates arsenic-induced liver fibrosis in mice. Exp Biol Med (Maywood) 233:377–384. doi:10.3181/0710-RM-269
Wu B, Liu S, Guo X, Zhang Y, Zhang X, Li M, Cheng S (2012) Responses of mouse liver to Dechlorane plus exposure by integrative transcriptomic and metabonomic studies. Environ Sci Technol 46:10758–10764
Yoshida T, Yamauchi H, Sun GF (2004) Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol 198:243–252. doi:10.1016/j.taap.2003.10.002
Zhou M et al (2012) Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J Proteome Res 11:4961–4971. doi:10.1021/pr3004826
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20131270), Foundation of State Key Laboratory of Pollution Control and Resource Reuse, and Science Foundation of Nanjing University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 7412 kb)
Rights and permissions
About this article
Cite this article
Hou, H., Yu, Y., Shen, Z. et al. Hepatic transcriptomic responses in mice exposed to arsenic and different fat diet. Environ Sci Pollut Res 24, 10621–10629 (2017). https://doi.org/10.1007/s11356-017-8743-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8743-9