Abstract
Introduction
In this study, we provide strategies for detecting and quantifying the structural isomers of polyfluorinated di- and tri-alkyl surfactants (PFAS) by mass spectrometry (MS). We specifically investigate polyfluorinated dialkylated phosphate ester surfactants (x:2/y:2 diPAPS, (F(CF2) x CH2CH2O-P(O)(O)−-OCH2CH2(CF2) y F)) and their thioether analogues (x:2/y:2 S-diPAPS, F(CF2) x CH2CH2SCH2-C[CH2O)2P(O)(O)−]-CH2SCH2CH2(CF2) y F), which are used for industrial applications, such as oil- and water-repellent coatings on paper and board. DiPAPS have been found in human blood and are metabolised to the persistent perfluoroalkyl carboxylic acids (PFCA) in rats.
Materials and methods
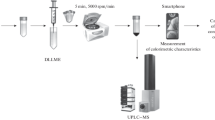
A microwave popcorn bag extract was analysed by ultrahigh-pressure liquid chromatography coupled to a negative electrospray ionisation-quadrupole time-of-flight MS.
Results and discussion
The extract contained S-diPAPS, diPAPS and trialkylated (triPAPS) impurities. TriPAPS were also present in industrial and synthetic diPAPS standards, and were verified with an 8:2/8:2/8:2 triPAPS standard. The eight elemental compositions (m/z’s) of diPAPS in the extract represent 19 precursor ion structures, and the six S-diPAPS m/z’s represent at least 13 structures. The diPAPS had [M-H]− precursor ions of m/z 789, 889,…1,489 and the S-diPAPS of m/z 921, 1,021,…1,421, corresponding to fluorinated chains from C6–18. Each m/z appeared as one to three chromatographic peaks of structural isomers, where, e.g. m/z 1,189 was present as 10:2/10:2, 8:2/12:2 and 6:2/14:2 diPAPS. The isomers formed different products ions, thus only half of the m/z 1,189 diPAPS concentration was measured with one precursor ion > product ion transition.
Conclusion
In general, knowledge about structural isomers of poly-alkylated PFAS is needed for the estimation of types and amounts of perfluorinated degradation products, such as PFCA from diPAPS.






Similar content being viewed by others
References
Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA (2005) Perfluorochemicals: potential sources of and migration from food packaging. Food Addit Contam 10:1023–1031, 9
Begley TH, Hsu W, Noonan G, Diachenko G (2008) Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit Contam Part A 25(3):384–390
Ciba (BASF) 2000–2010. http://www.ciba.com/ind-pap-eff-bar-lodyne.htm.Accessed 2 April 2010
D’eon JC, Mabury SA (2007) Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): exploring routes of human contamination. Environ Sci Technol 41(13):4799–4805
D’eon JC, Crozier PW, Furdui VI, Reiner EJ, Libelo EL, Mabury SA (2009) Observation of commercial fluorinated material, the polyfluoroalkyl phosphoric acid diesters, in human sera, wastewater treatment plant sludge, and paper fibers. Environ Sci Technol 43(12):4589–4594
De Silva AO, Muis DCG, Mabury SA (2009) Distribution of perfluorocarboxylate isomers in select samples from the north american environment. Environ Chem 28(9):1801–1814
E.I. du Pont de Nemours (2010) DuPont™ Zonyl fluorosurfactants (2001, (4/01) P-200125.8, No. H-49731-3. Printed in U.S.A.), and DuPont™ Zonyl fluorochemical intermediates (2001, (8/02) P-200125.8, No. H-49730-4, Printed in U.S.A.) and DuPont Foraperle. http://www2.dupont.com/. Accessed 14 May 2010
EC (2002) Commission Decision implementing Council Directive 96/23/EC concerning the performance of analytical methods and interpretation of results. 2002/657/EC. Official Journal of the European Communities L/221/8-36
European Committee for Standardization (2007) prEN 15519(2007). Paper and board intended to come into contact with foodstuffs—preparation of an organic solvent extract, E, CEN, Brussels
Guo J, Resnick P, Efimenko K, Genzer J, DeSimone JM (2008) Alternative fluoropolymers to avoid the challenges associated with perfluorooctanoic acid. Ind Eng Chem Res 47:502–508
Houde M, Czub G, Small JM, Backus S, Wang X, Alaee M, Muir DCG (2008) Fractionation and bioaccumulation of perfluorooctane sulfonate (PFOS) isomers in a Lake Ontario food web. Environ Sci Technol 42:9397–9403
Jensen AA, Leffers H (2008) Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 31:161–169, Review article
Kissa E (2002) Fluorinated surfactants, 2nd edn. Marcel Dekker, New York
Lange FT, Schmidt C, Brauch H-J (2006) Perfluoroalkylcarboxylates and sulfonates. Emerging contaminants for drinking water supplies? Association of river waterworks—RIWA. Available from: www.riwa-rijn.org/e_publikaties/137_ptfe_report.PDF. Accessed 12 June 2010
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Tox Sci 99(2):366–394
Lee H, D'eon J, Mabury SA (2010) Biodegradation of polyfluoroalkyl phosphates as a source of perfluorinated acids to the environment. Environ Sci Technol 44(9):3305–3310
Martin JW, Mabury SA, O’Brien PJ (2005) Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chem-Biol Interact 155:165–180
Mason Chemical Company (2010) Search for fluorinated surfactants. http://www.masonsurfactants.com. Accessed 14 April 2010
McLafferty FW, Turecek F (1993) Interpretation of mass spectra, 4th edn. University Science Books, Sausalito
Petrovic M, Hernando MD, Diaz-Cruz MSD, Barceló D (2005) Liquid chromatography-tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: a review. J Chrom A 1067:1–14
Tentschert J, Kappenstein O, Richter S, Luch A, Pfaff K (2010) Development of a screening method for perfluorinated compounds. Presented as poster at the 2nd fluoro surfactant workshop, 17–19 June 2010, Idstein, Germany
Trier X, Jackson D, Mabury SA, Christensen JH, Granby K, Weiner B (2010) Discovery of emerging fluorinated surfactants in Danish, Swedish and Canadian paper and boards for food contact by LC-MS and 19F NMR presentation and conference abstract at the fluorinated surfactants workshop, Idstein, Germany, 17–19 June 2010
Trier X, Granby K, Christensen JH (2011) Sources of polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Env Sci Pollut Res. doi:10.1007/s11356-010-0439-3
Acknowledgements
The environmental research school RECETO, Food-DTU and KU-LIFE funded the Ph.D. study, and Lundbeck and the Cowi foundations funded the UPLC system. Thanks to Masters student Lotte Kolind for work on the triPAPS HPLC-MS2 analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ake Bergman
Rights and permissions
About this article
Cite this article
Trier, X., Nielsen, N.J. & Christensen, J.H. Structural isomers of polyfluorinated di- and tri-alkylated phosphate ester surfactants present in industrial blends and in microwave popcorn bags. Environ Sci Pollut Res 18, 1422–1432 (2011). https://doi.org/10.1007/s11356-011-0488-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0488-2