Abstract
Purpose
To report the utility of positron emission tomography (PET) with α-[11C]methyl-l-tryptophan (AMT) for monitoring progression and response to treatment of an isolated optic pathway glioma (OPG) in a 16-year-old girl.
Procedures
Positron emission tomography scanning of the brain was performed 20 minutes after intravenous administration of AMT. The AMT-PET images were reconstructed and examined for tumor uptake of the tracer in correlation with coregistered magnetic resonance images.
Results
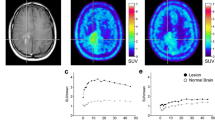
The PET scan demonstrated increased uptake of AMT by OPG in a clinically symptomatic child whose magnetic resonance imaging (MRI) was inconclusive for morphological changes of the tumor. The tracer uptake was dramatically decreased on the images obtained after chemotherapy. Subsequently, AMT-PET revealed a new tumor lesion of increased AMT uptake when the patient developed vision problems and MRI showed no significant interval morphological changes. Significant vision improvement was observed after external beam radiotherapy for the newly identified tumor lesion.
Conclusions
Positron emission tomography with α-[11C]methyl-l-tryptophan may be useful for monitoring progression and response to treatment of OPGs, which needs to be further investigated in a prospective study of more patients, including those with neurofibromatosis.


Similar content being viewed by others
References
Thiagalingam S, Flaherty M, Billson F, et al. (2004) Neurofibromatosis type 1 and optic pathway gliomas. Ophthalmology 111:568–577
Singhal S, Birch JM, Kerr B, et al. (2002) Neurofibromatosis type 1 and sporadic optic gliomas. Arch Dis Child 87:65–70
Hoffman HJ, Humphreys RP, Drake JM, et al. (1992) Optic pathway/hypothalamic gliomas: A dilemma in management. Pediatr Neurosurg 19:186–189
Listernick R, Louis DN, Packer RJ, Gutamann DH (1997) Optic pathway gliomas in children with neurofibromatosis 1: Consensus statement from the NF1 optic pathway glioma task force. Ann Neurol 41:143–149
Chateil JF, Soussotte C, Pedespan JM, et al. (2001) MRI and clinical differences between optic pathway tumors in children with and without neurofibromatosis. Br J Radiol 74:24–31
Benard F, Romsa J, Hustinx R (2003) Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med 33:148–162
Juhász C, Chugani DC, Muzik O, et al. (2006) In vivo uptake and metabolism of α-[11C]methyl-l-tryptophan in human brain tumors. J Cereb Blood Flow Metab 26:345–57
Bergstroem M, Litton J, Bohm C, Blomquist G (1982) Determination of object contour from projections for attenuation correction in cranial positron emission tomography. J Comput Assist Tomogr 6:365–72
Pietrzyk U, Herholz K, Fink G, et al. (1994) An interactive technique for three-dimensional image registration: Validation for PET, SPECT, MRI, and CT brain studies. J Nucl Med 35:2011–2018
Muzik O, Chugani DC, Chakraborty P, et al. (1997) Analysis of [C-11]alpha-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab 17:659–669
Chugani DC, Muzik O, Behen M, et al. (1999) Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol 45:287–295
Packer RJ, Lange B, Ater J, et al. (1993) Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol 11:850–856
Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL (1990) A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. J Cereb Blood Flow Metab 9:1–12
Chugani DC, Muzik O (2000) Alpha [C-11] methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab 20:2–9
Acknowledgment
We thank Pulak K. Chakraborty for preparation of the radiopharmaceutical AMT. This project was partly funded by a faculty research development fund awarded to F. P. by The Carman & Ann Adams Department of Pediatrics, School of Medicine, Wayne State University and a grant from the Children's Research Center of Michigan granted to C.J.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, F., Juhasz, C., Bhambhani, K. et al. Assessment of Progression and Treatment Response of Optic Pathway Glioma with Positron Emission Tomography using α-[11C]Methyl-l-Tryptophan. Mol Imaging Biol 9, 106–109 (2007). https://doi.org/10.1007/s11307-007-0090-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-007-0090-7