Abstract
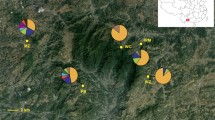
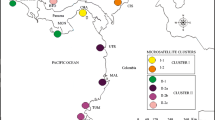
Topographic changes during the Pleistocene glacial/interglacial cycles affected the distribution of coastline mangroves and influenced their population genetic structure. The submergence of the continental shelf off southeast China during the postglacial age caused coastline expansions and resulted in the colonization of mangroves. Here, we performed multilocus genome scans using amplified fragment-length polymorphisms to explore the effects of topography and natural selection in structuring Kandelia obovata populations. Long-term isolation by the Taiwan Strait since the end of the last glacial maximum, which obstructed gene flow, differentiated the Taiwanese and Chinese populations. Founders that colonized from both outlets of the Taiwan Strait were sourced from the northern South China Sea and the Ryukyus, thereby creating a melting pot in the Taiwan Strait. Inner-strait currents played roles as vectors for propagule dispersal among populations. Upon examination of the allele-frequency distributions of outlier loci, most negative outliers reflected the widespread polymorphisms shared by common ancestors. Furthermore, significant differentiation in the genetic components of positive outliers between this and other populations and the negative correlation with geographic distance suggested the presence of geography- or latitude-independent population divergence. Restored populations were compared with their sources and revealed biased sampling of nursery seedlings, which caused within-population substructures and reduced effective population sizes. This study indicated that multiple factors affect the population structure of the mangroves off southeast China.




Similar content being viewed by others
References
Afzal-Rafii Z, Dodd RS, Fauvel MT (1999) A case of natural selection in Atlantic-East-Pacific Rhizophora. Hydrobiologia 413:1–9
Analuddin K, Sharma S, Suwa R, Hagihara A (2009) Crown foliage dynamics of mangrove Kandelia obovata in Manko Wetland, Okinawa Island, Japan. J Oceanogr 65:121–127
Antao T, Beaumont MA (2011) Mcheza: a workbench to detect selection using dominant markers. Bioinformatics 27:1717–1718
Antao T, Lopes A, Lopes RJ, Beja-Pereira A, Luikart G (2008) LOSITAN: a workbench to detect molecular adaptation based on a FST-outlier method. BMC Bioinforma 9:323
Barton N, Bengtsson BO (1986) The barrier to genetic exchange between hybridizing populations. Heredity 57:357–376
Beaumont MA, Balding DJ (2004) Identifying adaptive genetic divergence among populations from genome scans. Mol Ecol 13:969–980
Beaumont MA, Nichols RA (1996) Evaluating loci for use in the genetic analysis of population structure. Proc R Soc London, Ser B 263:1619–1626
Castillo-Cardenas MF, Toro-Perea N, Cardenas-Henao H (2005) Population genetic structure of Neotropical mangrove species on the Colombian Pacific Coast: Pelliciera rhizophorae (Pellicieraceae). Biotropica 37:266–273
Chen LZ, Wang WQ, Lin P (2004) Influence of water logging time on the growth of Kandelia candel seedlings. Acta Oceanologica Sinica 23:149–157
Chen SB, Ding WY, Qiu JB, Wang GY, Zhou ZM, Chen JF, Ai WM, Wang CY, Xie QL (2010) The genetic diversity of the mangrove Kandelia Obovata in China revealed by ISSR analysis. Pak J Bot 42:3755–3764
Chen SF, Zhou RC, Huang YL, Zhang M, Yang GL, Zhong CR, Shi SH (2011) Transcriptome sequencing of a highly salt tolerant mangrove species Sonneratia alba using Illumina platform. Marine Genomics 4:129–136
Chiang TY (2000) Lineage sorting accounting for the disassociation between chloroplast and mitochondrial lineages in oaks of southern France. Genome 43:1090–1094
Chiang TY, Chiang YC, Chen YJ, Chou CH, Havanond S, Hong TN, Huang S (2001) Phylogeography of Kandelia candel in East Asiatic mangroves based on nucleotide variation of chloroplast and mitochondrial DNAs. Mol Ecol 10:2697–2710
Deng SL, Huang YL, He HH, Tan FX, Ni XW, Jayatissa LP, Hettiarachi S, Sh SH (2009) Genetic diversity of Aegiceras corniculatum (Myrsinaceae) revealed by amplified fragment length polymorphism (AFLP). Aquat Bot 90:275–281
Dodd RS, Afzal-Rafii Z, Kashani N, Budrick J (2002) Land barriers and open oceans: effects on gene diversity and population structure in Avicennia germinans L. (Avicenniaceae). Mol Ecol 11:1327–1338
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15
Duke NC, Ball MC, Ellison JC (1998) Factors influencing biodiversity and distributional gradients in mangroves. Glob Ecol Biogeogr Lett 7:27–47
Duke NC, Lo EYY, Sun M (2002) Global distribution and genetic discontinuities of mangroves—emerging patterns in the evolution of Rhizophora. Trees-Structure and Function 16:65–79
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361. doi:410.1007/s12686-011-9548-7
El-Kassaby YA, Thomson AJ (1996) Parental rank changes associated with seed biology and nursery practices in Douglas-fir. For Sci 42:228–235
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Hofer T, Foll M (2009) Detecting loci under selection in a hierarchically structured population. Heredity 103:285–298
Excoffier L, Ray N (2008) Surfing during population expansions promotes genetic revolutions and structuration. Trends Ecol Evol 23:347–351
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Geng QF, Lian CL, Goto S, Tao JM, Kimura M, Islam MDS, Hogetsu T (2008) Mating system, pollen and propagule dispersal, and spatial genetic structure in a high-density population of the mangrove tree Kandelia candel. Mol Ecol 17:4724–4739
Giang LH, Geada GL, Hong PN, Tuan MS, Lien NTH, Ikeda S, Harada K (2006) Genetic variation of two mangrove species in Kandelia (Rhizophoraceae) in Vietnam and surrounding area revealed by microsatellite markers. Int J Plant Sci 167:291–298
Guo W, Li SH, Mao L, Yin Y, Zhu DK (2007) A model for environmental impact assessment of land reclamation. China Ocean Eng 21:343–354
Gwada P, Makoto T, Uezu Y (2000) Leaf phenological traits in the mangrove Kandelia candel (L.) Druce. Aquat Bot 68:1–14
Hallatschek O, Hersen P, Ramanathan S, Nelson DR (2007) Genetic drift at expanding frontiers promotes gene segregation. Proc Natl Acad Sci U S A 104:19926–19930
Hamilton MB (2009) Chapter 8: molecular evolution. In: population genetics. Wiley-Blackwell, Oxford, pp 235–282
Harlan JR (1975) Our vanishing genetic resources. Science 188:618–621
Hogue ATMR, Sharma S, Suwa R, Mori S, Hagihara A (2010) Seasonal variation in the size-dependent respiration of mangroves Kandelia obovata. Mar Ecol Prog Ser 404:31–37
Huang YL, Tan FX, Su GH, Deng SL, He HH, Shi SH (2008) Population genetic structure of three tree species in the mangrove genus Ceriops (Rhizophoraceae) from the Indo West Pacific. Genetica 133:47–56
Huang ZY, Yu HS (2003) Morphology and geologic implications of Penghu Channel off southwest Taiwan. Terr Atmos Ocean Sci 14:469–485
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332
Jan S, Wang J, Chern CS, Chao SY (2002) Seasonal variation of the circulation in the Taiwan Strait. J Mar Syst 35:249–268
Jorde PE, Palm S, Ryman N (1999) Estimating genetic drift and effective population size from temporal shifts in dominant gene marker frequencies. Mol Ecol 8:1171–1178
Kado T, Fujimoto A, Giang LH, Tuan M, Hong PN, Harada K, Tachida H (2004) Genetic structures of natural populations of three mangrove species, Avicennia marina, Kandelia candel and Lumnitzera racemosa, in Vietnam revealed by maturase sequences of plastid DNA. Plant Species Biol 19:91–99
Kao WY, Shih CN, Tsai TT (2004) Sensitivity to chilling temperatures and distribution differ in the mangrove species Kandelia candel and Avicennia marina. Tree Physiol 24:859–864
Kitaya Y, Jintana V, Piriyayotha S, Jaijing D, Yabuki K, Izutani S, Nishimiya A, Iwasaki M (2002) Early growth of seven mangrove species planted at different elevations in a Thai estuary. Trees-Structure Funct 16:150–154
Klopfstein S, Currat M, Excoffier L (2006) The fate of mutations surfing on the wave of a range expansion. Mol Biol Evol 23:482–490
Kokita T, Nohara K (2011) Phylogeography and historical demography of the anadromous fish Leucopsarion petersii in relation to geological history and oceanography around the Japanese Archipelago. Mol Ecol 20:143–164
Liao HR, Yu HS, Su CC (2008) Morphology and sedimentation of sand bodies in the tidal shelf sea of eastern Taiwan Strait. Mar Geol 248:161–178
Liao PC, Chiang YC, Huang S, Wang JC (2009) Gene flow of Ceriops tagal (Rhizophoraceae) across the Kra Isthmus in the Thai Malay Peninsula. Bot Stud 50:193–204
Liao PC, Havanond S, Huang S (2007) Phylogeography of Ceriops tagal (Rhizophoraceae) in Southeast Asia: the land barrier of the Malay Peninsula has caused population differentiation between the Indian Ocean and South China Sea. Conserv Genet 8:89–98
Liao PC, Hwang SY, Huang S, Chiang YC, Wang JC (2011) Contrasting demographic patterns of Ceriops tagal (Rhizophoraceae) populations in the South China Sea. Aust J Bot 59:523–532
Liao PC, Kuo DC, Lin CC, Ho KC, Lin TP, Hwang SY (2010) Historical spatial range expansion and a very recent bottleneck of Cinnamomum kanehirae Hay. (Lauraceae) in Taiwan inferred from nuclear genes. BMC Evol Biol 10:124
Lin CC (1963) Geology and ecology of Taiwan prehistory. Asian Perspect 7:203–213
Manel S, Conord C, Despres L (2009) Genome scan to assess the respective role of host-plant and environmental constraints on the adaptation of a widespread insect. BMC Evol Biol 9:288
McKay JK, Bishop JG, Lin JZ, Richards JH, Sala A, Mitchell-Olds T (2001) Local adaptation across a climatic gradient despite small effective population size in the rare sapphire rockcress. Proc R Soc B Biol Sci 268:1715–1721
Meirmans PG (2012) The trouble with isolation by distance. Mol Ecol 21:2839–2846
Melville F, Burchett M (2002) Genetic variation in Avicennia marina in three estuaries of Sydney (Australia) and implications for rehabilitation and management. Mar Pollut Bull 44:469–479
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A 76:5269–5273
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Saenger P (1998) Mangrove vegetation: an evolutionary perspective. Mar Freshw Res 49:277–286
Sheue CR, Liu HY, Yong JWH (2003) Kandelia obovata (Rhizophoraceae), a new mangrove species from Eastern Asia. Taxon 52:287–294
Su GH, Huang YL, Tan FX, Ni XW, Tang T, Shi SH (2006) Genetic variation in Lumnitzera racemosa, a mangrove species from the Indo-West Pacific. Aquat Bot 84:341–346
Sun M, Wong KC, Lee JSY (1998) Reproductive biology and population genetic structure of Kandelia candel (Rhizophoraceae), a viviparous mangrove species. Am J Bot 85:1631–1637
Thiel M, Haye PA (2006) The ecology of rafting in the marine environment. III. Biogeographical and evolutionary consequences. Oceanogr Mar Biol Annu Rev 44:323–429
Triest L (2008) Molecular ecology and biogeography of mangrove trees towards conceptual insights on gene flow and barriers: A review. Aquat Bot 89:138–154
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Ye Y, Gu YT, Gao HY, Lu CY (2010) Combined effects of simulated tidal sea-level rise and salinity on seedlings of a mangrove species, Kandelia candel (L.) Druce. Hydrobiologia 641:287–300
Ye Y, Wong YS, Tam NFY (2005) Acclimation of a dominant mangrove plant (Kandelia candel) to soil texture and its response to canopy shade. Hydrobiologia 539:109–119
Yin W, Fu CZ, Guo L, He QX, Li J, Jin BS, Wu QH, Li B (2009) Species delimitation and historical biogeography in the genus Helice (Brachyura: Varunidae) in the Northwestern Pacific. Zool Sci 26:467–475
Yu H-S, Song G-S (1993) Submarine physiography around Taiwan and its relation to tectonic setting. J Geol Soci China 36:139–156
Yu HS (2003) Geological characteristics and distribution of submarine physiographic features in the Taiwan region. Mar Georesour Geotechnol 21:139–153
Yu HS, Chou YW (2001) Physiographic and geological characteristics of shelves in north and west of Taiwan. Sci China Ser D Earth Sci 44:696–707
Zhou RC, Ling SP, Zhao WM, Osada N, Chen SF, Zhang M, He ZW, Bao H, Zhong CR, Zhang B, Lu XM, Turissini D, Duke NC, Lu J, Shi SH, Wu CI (2011) Population genetics in nonmodel organisms: II. Natural selection in marginal habitats revealed by deep sequencing on dual platforms. Mol Biol Evol 28:2833–2842
Acknowledgments
Funds are partially supported by National Science Council, R.O.C (NSC 99-2621-B-020-002-MY3) to P.-C. Liao.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Aitken
Y. Ruan and B.-H. Huang contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 374 kb)
Rights and permissions
About this article
Cite this article
Ruan, Y., Huang, BH., Lai, SJ. et al. Population genetic structure, local adaptation, and conservation genetics of Kandelia obovata . Tree Genetics & Genomes 9, 913–925 (2013). https://doi.org/10.1007/s11295-013-0605-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-013-0605-0