Abstract
Large-scale exploitation of higher trophic levels by humans, together with global-scale nutrient enrichment, highlights the need to explore interactions between predator loss and resource availability. The hypothesis of exploitation ecosystems suggests that top–down and bottom–up control alternate between trophic levels, resulting in a positive relationship between primary production and the abundance of every second trophic level. Specifically, in food webs with three effective trophic levels, primary producers and predators should increase with primary production, while in food webs with two trophic levels, only herbivores should increase. We provided short-term experimental support for these model predictions in a natural benthic community with three effective trophic levels, where the number of algal recruits, but not the biomass of gastropod grazers, increased with algal production. In contrast, when the food web was reduced to two trophic levels by removing larger predators, the number of algal recruits was unchanged while gastropod grazer biomass increased with algal production. Predator removal only affected the consumer-controlled early life-stages of algae, indicating that both the number of trophic levels and the life-stage development of the producer trophic level determine the propagation of trophic cascades in benthic systems. Our results support the hypothesis that predators interact with resource availability to determine food-web structure.
Similar content being viewed by others
Introduction
Changes in ecosystem configuration following declines in higher trophic level predators are often explained by fitting a simple trophic cascade model to time-series data of a limited number of species (e.g., McLaren and Peterson 1994; Estes et al. 1998; Heithaus et al. 2008). However, the top–down control of food web dynamics depends on complex interactions between environmental conditions, resource supply, individual species traits, and food web complexity (Polis and Strong 1996; Polis 1999; Menge 2000; Oksanen and Oksanen 2000; Shurin et al. 2002; Hopcraft et al. 2010). These interactions indicate that predator effects on food web configuration are highly context dependent and that ecosystem-specific properties determine responses to exploitation of higher trophic levels (Strong 1992; Shurin et al. 2002; Hopcraft et al. 2010). Thus, to further understand the impact of predators on food-web structure and be able to predict effects of predator declines, we need to test predator effects in combination with both abiotic and biotic properties of natural food webs.
The hypothesis of exploitation ecosystems (EEH) predicts that community level trophic cascades interact strongly with the productivity of the ecosystem (Fretwell 1977; Oksanen et al. 1981; Oksanen and Oksanen 2000). According to the EEH (Oksanen et al. 1981), top–down control of herbivores and bottom–up control of primary producers should dominate in high-productivity areas, generating a positive relationship between productivity, primary producer biomass, and carnivores. In contrast, in low-productivity areas that cannot energetically support carnivores, the bottom–up control of herbivores and top–down control of primary producers should generate a positive relationship between productivity and herbivores (Oksanen and Oksanen 2000). However, taking density-dependent interactions between consumers (ratio-dependent predation) into account suggests that the strength of trophic cascades rapidly diminishes down trophic levels when intraguild interactions increase (Herendeen 1995; Ives et al. 2005). At the same time, bottom–up effects from resource enrichment can propagate up the food web with an almost unchanged strength when prey dependence is high (Herendeen 1995). The dominance of bottom–up effects in food webs with strong intraguild interactions are supported by a number of enrichment experiments on different spatial and temporal scales in which the total biomass of all trophic levels increased when resources were added (e.g., Carpenter et al. 2001; Persson et al. 2001). Thus, mixed top–down and bottom–up effects according to the EEH may depend both on strong prey dependence of consumers and on weak intraguild interactions within consumer trophic levels (Herendeen 2004).
The EEH is also a stable equilibrium theory that assumes long-term population dynamics, a closed system, and spatially homogenous habitats, all of which may critically limit predictions for short-term experiments testing the effects of removing predators. Accordingly, field studies support a positive relationship between primary production and trophic cascades in some systems (e.g., Sinclair et al. 2000; Elmhagen and Rushton 2007; Moksnes et al. 2008; Eriksson et al. 2009; Sieben et al. 2011), whereas a recent set of meta-analyses suggests that, in general, it is the traits of consumer species, and not productivity, that determine the prevalence of trophic cascades in predator removal experiments (Borer et al. 2005, 2006). However, models that incorporate short-term population dynamics and allow for dispersal suggest that the an alternating positive relationship between trophic levels and primary productivity should also appear in environments where consumers immigrate and emigrate actively between patches depending on resource availability (Wootton and Power 1993; Oksanen et al. 1995; Nisbet et al. 1997). A key condition for trophic cascades in these models is that top-predators must employ active dispersal and distribute freely, while passive dispersal generates a positive relationship between primary productivity and all trophic levels (Nisbet et al. 1997).
In the study reported here, we tested whether predators change the relationship between primary production and the abundance of algae and grazers by excluding all larger predators and manipulating resource availability in a benthic food web. Our study system was dominated by highly mobile invertebrate grazers and predators. There is also a strong relationship between vulnerability to grazing and life-stage, such that grazers negatively affect macroalgal recruitment by consuming smaller life-stages, while already established and larger macroalgae are mainly limited by nutrient and light availability (Lotze and Worm 2000; Lotze et al. 2000; Worm et al. 2001; Eriksson et al. 2006). Thus, we assumed that resource availability control the biomass production of established macroalgae and that consumers control new macroalgal recruits. We tested the relationship between algal production and the abundance of algal recruits and grazers as a function of the number of trophic levels. We hypothesized that: (1) the number of algal recruits (as a relative measure of standing biomass) would increase with net production in the presence of predators, but not in the absence of predators, and (2) the biomass of grazers would increase with net production in the absence of predators, but not in the presence of predators.
Materials and methods
Study site
The experiment was conducted from April to August 2006 at Maasholm in the brackish Schlei Fjord, southern Baltic Sea, Germany (54°41′N, 10°0′E). The Schlei Fjord is characterized by insignificant tides, and in the summer, the salinity ranges between 12 and 18 PSU (practical salinity units), nutrient concentrations fluctuate highly, and water temperatures range between 16 and 25°C (Worm and Lotze 2006). The sandy benthos scattered with rocks support a submerged macroalgal community dominated by the canopy-forming macroalgae Fucus vesiculosus (hereafter Fucus), which covers approximately 80% of the stone surface along with several crust-forming species and a number of fast-growing green ephemerals (Eriksson et al. 2006). The grazer community consists of small peracarid crustacean mesograzers (isopods and amphipods) and 1- to 2-cm-sized snails (mainly periwinkles, Littorina littorea, and L. saxatilis). The local predator community in the experimental area is dominated by crabs (Carcinus maenas), but also includes small benthic fish (mainly Gobius niger and Pomatoschistus minutus). Carcinus maenas is an omnivore that consumes animals, plant material, and algae. However, the diet of adult individuals mainly comprises molluscs, especially the common mussel (Mytilus edulis), dogwhelk (Nucella lappilus), and periwinkle (Rangeley and Thomas 1987; Little and Kitching 1996). Therefore, in the experiment area is C. maeneas a potential predator on gastropod grazers, while the benthic fish mainly eat the small crustacean mesograzers. Thus, the local community has three effective trophic levels, while stationary higher top-predators are scarce (personal observation). All of the common consumers are highly mobile and easily moved between experimental plots (indicating an active rather than passive distribution).
Grazer control of the studied macroalgae community is strongest for the early life-stages of algae, while herbivores have only minor effects on the adult community under natural conditions (Lotze and Worm 2000; Worm et al. 2001). Fucus reproduce by simultaneously releasing gametes on a few occasions in late spring–early summer, while the dominating ephemeral green algae reproduce by releasing propagules continuously during the entire spring and summer. A number of grazer exclosure experiments performed previously in the study area revealed that these early life-stages are highly susceptible to grazing, especially during the first 14 days after settlement (Lotze and Worm 2000; Worm et al. 2001 ; Eriksson et al. 2007). However, many of the algae produce a variety of microscopic resting stages that overwinter on the surfaces of rocks (Chapman 1986; Eriksson and Johansson 2005). These microscopic life-stages act as an overwintering propagule bank from which the algae can regenerate; they thereby enable a significantly earlier onset of growth in spring and provide a seasonal escape from high levels of grazing later in the year (Kiirikki and Ruuskanen 1996; Lotze et al. 2000; Worm et al. 2001; Eriksson et al. 2006). Once the algal propagules start to accumulate visible biomass, they are much less susceptible to grazers (Lotze and Worm 2000; Worm et al. 2001). Accordingly, earlier experiments at the study site demonstrated that the production of algal biomass is controlled by light availability in a quadratic relation (Pearson moment correlation of bottom light availability and the square root of the macroalgal biomass accumulated over summer on propagule-seeded bricks in 2004 and 2005: n = 42, t, p, R 2 = 0.58; Fig. 1) and that nutrients increase biomass production only at high light (Eriksson et al. 2006, 2007). Thus, the square root of biomass development of already established macroalgal propagules should therefore provide a good relative measure of resource availability and primary production at our field site (Eriksson et al. 2006), while the abundance of recruits should represent the fraction of the algal community sensitive to consumer control. It is also reasonable to assume that resource availability estimated from biomass development of the already established macroalgal propagules is also a good estimation of the resource availability for the newly established propagules.
The relation between light availability and the net production of macroalgal biomass during the summer at the field site. Measurements were made on substrates cleaned of adult vegetation at the beginning of the experimental period. Data are from earlier experiments in 2004 (circles) and 2005 (crosses) (Eriksson et al. 2006, 2007). Sqrt Square root, dw dry weight
Field methods
How the number of trophic levels and resource availability influence the abundance of algae and grazers, respectively, was studied by experimentally manipulating predator presence, light availability, and nutrients in the field. Predator presence was manipulated using exclusion cages (40 × 40 × 40 cm) covered with a green plastic garden net. The 2.0 × 2.0-cm mesh size allowed amphipod and isopod mesograzers, periwinkles, and small crabs to enter the cages, but excluded adult crabs and fish predators. To separate cage effects from true predator effects, we placed closed cages that excluded predators but allowed grazers to enter (‘closed cage’ treatment) on one-third of the plots, cages with 15 × 15-cm holes (‘open cage’ treatment) that allowed predators to enter on another one-third of the plots, and no cages (‘no cage’ treatment) on the last one-third of the plots. Cages were cleaned from fouling organisms once every 2 weeks, when we also inspected the closed cages for invading fish and crabs. Throughout the experimental period, only occasional juvenile crabs were found inside the closed cages, but we did not remove them since they were too small to be excluded by the mesh.
Light availability was manipulated by placing a canopy cover of Fucus individuals in half of the plots (‘canopy’ vs. ‘no canopy’ treatment). The Fucus was attached naturally to a small rock that was placed approximately 15 cm next to the sampling substrates (inside the cage when cages were present). The stones were placed in the water approximately 2.5 years earlier to allow a thick Fucus cover of similar height to accumulate. We selected canopies that were not reproductive at the experimental start and found no developing reproductive parts on algae in the canopy treatment throughout the year. Canopies also provide a habitat for invertebrate grazers, but earlier experiments showed that canopy presence has no significant effect on the abundances of associated invertebrates or grazing intensity on neighboring understory substrates, such as the sampling substrates used to sample grazers and algae in this experiment (see experiment design below) (Eriksson et al. 2007). Instead, canopies control the accumulation of macroalgal biomass by limiting light (Eriksson et al. 2006, 2007).
Nutrient resources were manipulated by enriching half of the plots with 120 g of slow release NPK-fertilizer pellets (Plantacote Depot 6 M; Urania Agrochem, Hamburg, Germany) (‘nutrient enriched’ vs. ‘ambient’ conditions). Fertilizer pellets were supplied in plastic nets that were changed every 5–6 weeks. At our field site, this method elevates summer concentrations of inorganic nitrogen by 20–25% and phosphorus by 20–40% in the water around the plots (Worm et al. 2000; Eriksson et al. 2006, 2007). Plots were separated by at least 3 m to avoid spill-over from the NPK treatment to adjacent plots.
The experiment was designed in a factorial combination of predator exclusion (closed cage/open cage/no cage), light availability (canopy/no canopy), and nutrients (nutrient enrichment/ambient nutrients). Treatments were arranged in a random block design; three blocks were placed parallel to the shore, with each block containing one randomly distributed replicate of all treatment combinations (replicates = 3, total n = 36). The experiment was started on 10 April 2006; in each plot (5 × 5 cm), one ceramic tile was placed to sample new algal recruits and a clay brick (10 × 20 cm) with a well-developed propagule bank was placed to sample experimental effects on already established vegetation and macrofauna grazers. The macroalgae/fauna brick had been placed in the water approximately 1.5 years earlier (October 2004), and prior to the experiment in 2006 it was scraped to remove all macroscopic vegetation. Algal recruit tiles were sampled on 17 June 2006 and the number of recruits counted under a stereomicroscope in the laboratory. Due to the microscopic size of the recruits, the number of recruits is a more precise measure of abundance than biomass because contamination by inorganic particles from the substrate or tube-building crustaceans has a major influence on the dry weight of sampled recruit populations. Since the recruits were sampled on sterile substrates during a short time window, they were of similar sizes, and therefore the number of recruits could be used as a good relative measure of biomass. Fauna abundance and macroalgal biomass production on the propagule bricks were sampled on 29 August 2006 by wrapping a plastic bag around the brick in situ and transporting it to the laboratory. All macrofauna and macroalgae were identified to the species level, counted, and dried at 80°C for 48 h for dry weight measurements. Fauna within the Fucus canopy was not assessed because the abundance, biomass, and diversity of invertebrates increase with canopy volume (Eriksson et al. 2007). The densities of crabs (Carcinus maenas) and fish in the plots were determined by snorkeling. We found no fish in the plots, and the biomass of the grazer fauna was completely dominated by periwinkle (Littorina spp.; see Results). Crabs and periwinkle are the most conspicuous benthic consumers in the area, but the sampling methods may also have favored a better representation of these groups since small crustaceans and fish have very fast escape behaviors. Since crabs and periwinkles dominated the fauna and represent a trophic chain of consumers and prey—periwinkles are a preferential food source for crabs (Rangeley and Thomas 1987; Little and Kitching 1996)—we used periwinkle biomass to represent the grazer trophic levels in subsequent analyses.
Data analyses
Treatment effects on macroalgal production (biomass development of all macroalgae on the propagule seeded bricks), and the abundance of macroalgal recruits (number of all macroalgae recruits on the sterile tiles), biomass of gastropod grazers (dry weight of periwinkle found on the bricks), and number of crab predators in the plots were analyzed using general linear mixed (GLM) models. The models included full factorial combinations of the fixed factors predator exclusion, canopy presence, and nutrient enrichment; the random factor block was used as a non-interacting explanatory variable. If necessary, data were log 10 or square root transformed (if most values were <1) to meet the criteria of homogeneous variances according to Cochran’s test. To specifically test the relationship between productivity and standing biomass at the algae and grazer trophic levels, we used the biomass development of macroalgae on the propagule-seeded bricks as a relative measure of resource availability and macroalgal production (see Materials and methods for rationale) and the number of algal recruits as a measure of the standing biomass of the fraction of the algal community actually targeted by grazers. We therefore constructed a new set of GLM models in which we described the number of macroalgal recruits and gastropod grazer biomass using the factorial combination of predator presence (fixed factor) and macroalgal production estimated from the square root of the measurement of biomass development on the propagule-seeded bricks (continuous variable) as explanatory variables. Note that growth of the added canopy was not included in the macroalgal production. Again, block was included as a random factor. Thus, we replaced the fixed resource treatments (nutrient and light manipulations) with a continuous proxy for resource availability and primary production. Here, we also pooled the ‘no cage’ and ‘open cage’ treatments. Together, this allowed us to more specifically test the relationships between productivity and standing biomass in food webs with two or three trophic levels. Two samples were lost/destroyed in the field, and these plots were excluded from all analyses.
Results
The stationary predator community was completely dominated by crabs; no fish were ever found in any of the plots. The biomass of the invertebrate grazer community comprised 99.9% gastropod grazers (periwinkle, Littorina spp.). Macroalgal production as measured by algal biomass on the propagule-seeded bricks was dominated by Fucus vesiculosus (95.8%), while the number of recruits counted on the sterile substrates was evenly distributed between F. vesiculosus and the green algae Cladophora glomerata. Since the periwinkle grazes unselectively by scraping the substrate in its path clean, we used the total number of macroalgal recruits in the analyses.
Nutrient availability clearly controlled macroalgal production, while the predator treatments did not influence algae or gastropod grazers. Nutrient enrichment increased the biomass production of macroalgae on the propagule bricks by 50%, but neither the predator nor canopy treatments had any significant effect on macroalgal biomass (Table 1, ‘all fixed effects’). Removal of the predator and resource availability treatments (light regulation and nutrient enrichment) did not affect the number of macroalgal recruits or the biomass of gastropod grazers (Table 1, ‘all fixed effects’). Crab abundances decreased from an average of one crab in 91.3% of the ‘open cage’ or ‘no cage’ plots, to one in 17% of the ‘closed cage’ plots. However, the crabs that invaded the closed cages were only two smaller juveniles, while the others were all larger adults. Thus, the closed cages indeed excluded larger fauna (the bulk of predators in the system), and there was no significant cage effect on any of the response variables (Table 1, ‘all fixed effects’; cage effects for crabs, mixed GLM: ‘open cage’ vs. ‘no cage’ F 1,19 = 0.22, p = 0.647; block F 2,19 = 1.96, p = 0.168).
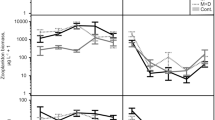
The number of trophic levels (presence or not of predator) had significant effects on both the total number of macroalgal recruits and the total biomass of gastropod grazers by changing the relation between the number of algal recruits, gastropod biomass, and macroalgal production. For the number of macroalgal recruits, the interaction effect between predator presence and macroalgal production in the GLM model showed a marginally significant trend (p = 0.068; Table 1, ‘biomass production as covariate’), but there was a strong positive linear correlation between the density of recruits and macroalgal production with three trophic levels present (Fig. 2a; Pearson moment correlation, n = 23, r = 0.62, p = 0.001) that disappeared when the predators were removed (two trophic levels present) (Fig. 2b; n = 11, r = 0.00, p = 0.994). For the biomass of gastropod grazers, there was a strong interaction between predator presence and macroalgal production in the GLM model (Table 1, ‘biomass production as covariate’). Here, seemingly unrelated distributions of grazer biomass and macroalgal production (Fig. 2c; n = 23, r = 0.01, p = 0.961) turned into a positive linear relation when the predators were removed (Fig. 2d; n = 11, r = 0.62, p = 0.042).
The relation between the net production of algal biomass and the number of algal recruits in the presence of predators (a) (3 trophic levels; open circles open cages, crosses no cages), the number of algal recruits in the absence of predators (b) (2 trophic levels; filled circles closed cages), biomass of gastropod grazers in the presence of predators (c) (3 trophic levels; open circles open cages, crosses no cages), and biomass of gastropod grazers in the absence of predators (d) (2 trophic levels; filled circles closed cages)
Discussion
The results of this study demonstrate that the biomass of organisms occupying lower trophic levels depends both on the number of trophic levels and the production of the system, which are in line with the predictions from prey-dependent models of both long-term (EEH) (Oksanen et al. 1981; Oksanen and Oksanen 2000) and short-term population dynamics (Nisbet et al. 1997). Consumer-controlled compartments of the algal community, i.e., the number of algal recruits, increased with algal production when three trophic levels were present in the food web, while gastropod grazer biomass increased with algal production only when the food web was reduced to two trophic levels with the removal of the larger predators. Such trophic cascades develop in models which assume that feeding rates are dependent on the number of prey only (prey-dependent models), while models that also take into account consumer densities and intra-guild interactions (ratio dependence) do not predict an alternating accumulation of biomass over trophic levels (Abrams and Ginzburg 2008; Herendeen 2004). Our results suggest that the consumption rates at natural densities of consumers and prey were mainly determined by prey availability during our experiment, while the effect on consumption rates by intraspecific interference was negligible. To evaluate if the prey-dependent functional response is general to the marine benthos, we need to explore the consumption behavior of other groups of mesograzers and include the natural background densities of both grazers and predators from other areas into the experimental design.
The positive effect of the combination of production and predator presence on the density of macroalgal recruits follows robust mathematical theory incorporating short-term population dynamics. This theory predicts that for a variety of assumptions and models, producer abundances increase due to both increased resource availability and predator immigration rates and decrease from increased grazer immigration rates (Nisbet et al. 1997). Prey-dependent models of short-term population dynamics also predict a general increase in grazer abundances with increased producer production (Nisbet et al. 1997), but this relationship only degenerates in the presence of predators in models where predators actively disperse depending on resource availability (e.g., Wootton and Power 1993). At the scale of our experiment, all main predators moved freely between the treatments and showed aggregation in habitat patches with vegetation, clearly demonstrating that predators did not distribute passively. Interestingly, a survey of manipulative experiments revealed that the marine benthos is the only ecosystem in which grazers generally show an increase with nutrient enrichment and where cascading effects on lower trophic levels from predator removal are the strongest (Borer et al. 2005, 2006; Gruner et al. 2008). Thus, the marine benthos seems to be vulnerable to cascading behavior between trophic levels, which may be promoted by the common conditions of highly mobile predators and a dominance of continuously reproducing and well-mixed distributions of producers (Nisbet et al. 1997).
Another observation on the spatial dynamics aspect of our experiment, in which consumers could move between patches of algal resources, is that the algal response was a population level response, while the consumer response was mainly an individual level response. This means that algal recruits were controlled by consumer-induced mortality or resource-limited growth, while the response of the gastropods may also have been behaviorally mediated since they were able to escape predators or low-resource conditions by migrating. Behaviorally mediated indirect effects of both resource availability and predation risk often contribute strongly to numerical effects on prey density (Heithaus et al. 2008), likely strengthening the effects on the gastropod grazers in our experiment since there were numerous alternative predator-free resource patches for them to escape to. This is a significant difference compared to the original EEH by Oksanen et al. (1981) which is an equilibrium theory with all trophic levels at their population capacity. However, Nisbet et al. (1997) showed that for temporal and spatial scales typically employed in field experiments, population- and individual-based models produce similar results as long as they allow for active dispersal of the consumers.
Predator declines often initiate species cascades (changes in a few species of dominating prey), while examples of true trophic cascades with changes in total trophic level abundances are more rare (Strong 1992; Polis 1999; Polis et al. 2000). The prevalence of trophic cascades depends on strong consumer control of the prey community, with a cascading indirect positive effect being generated on the next lower trophic level. There are a number of distinct species traits that determine if species are subjected to consumer control (Duffy and Hay 1990; Coley and Barone 1996; Polis 1999; Hopcraft et al. 2010), and the response of the community to changes in predators should ultimately depend on the community composition of each trophic level (Gruner et al. 2008). Our results support the concept that the life-stage development of the producer trophic level can be essential for responses to predator declines in the marine benthos, by demonstrating that established larger macroalgae were resource controlled regardless of the composition of the higher trophic levels. In addition, the significant effects on food-web structure by predation were apparent only when biomass production was included as an underlying variable in the analyses. This finding emphasizes the need to explore the interdependence between complex ecological interactions, such as joint effects of top–down and bottom–up control, by incorporating environmental conditions and resource availability in food-web analyses (Olff et al. 2009; Hopcraft et al. 2010). Thus, we cannot understand ecosystem effects of predator exploitation and resource loading by only monitoring standing stocks.
Life-stage dependence of vulnerability to consumers is common in marine systems, where many animals actually grow through the trophic levels. For example, benthic fish may start out by eating zooplankton as larvae and then herbivorous crustaceans as larger juveniles, and then move to include other predatory fish as they grow in size (Werner and Gilliam 1984; Winemiller 1989). For primary producers, the vulnerability of small life-stages may be emphasized on rocky shores because once germlings on rocky substratum are removed, they cannot regenerate from any belowground parts. Many of the dominating grazers, such as urchins or gastropods, also feed by scraping the rocky surface clean with specialized teeth, leaving little organic material on which algae can regenerate. Thus, grazing on an early life-stage of an algae most likely leads to this individual being removed from the population. At the same time, macroalgae usually outgrow their grazers by several magnitudes in size, and once the algae reach a certain size they are therefore unlikely to be killed by their consumers, although this did happen occasionally in our study system during mass outbreaks of grazers. In addition, the highly undifferentiated thallus of most algae means that grazed parts of adult tissue can quickly regenerate, probably contributing to the weakened consumer effects on adult algae. Thus, the importance of life-stages on food-web dynamics may be emphasized in marine systems. However,, body size is also a fundamental food-web trait in terrestrial habitats. For example, in savanna ecosystems, herbivore body size together with plant nutritional quality, determine the relative strength of bottom–up and top–down control, which in turn have strong feed-backs on vegetation type (Hopcraft et al. 2010).
Increasing globalization and intensification of human impacts highlight the need to understand which properties of food webs contribute to the resilience of ecosystem functions. Here, we provide evidence that predator communities and the number of trophic levels significantly affect community responses to resource loading. Our results demonstrate the importance of higher trophic levels on the behavior of lower trophic levels following exposure to human disturbances. Furthermore, the fact that we were able to demonstrate a significant trophic cascade on the recruitment stages of algae indicates that a lack of a clearly visible community-wide trophic cascade following declines in predator abundance in nature does not exclude large, community-wide effects on a longer time scale. Such delayed effects have been demonstrated for a rocky shore mesocosm community, where nutrient enrichment and changes in grazer abundances generated a total restructuring of the macroalgal community after 4 years of limited effects (Kraufvelin et al. 2006). Thus, while short-term community-wide trophic cascades due to predator removal may not be common in nature, effects of predator declines may cascade down the food web through changes in community composition and thereby interact with nutrient loading over the long term (Eriksson et al. 2009; Sieben et al. 2011).
References
Abrams PA, Ginzburg LR (2008) The nature of predation: prey dependent, ratio dependent or neither? Trends Ecol Evol 2000:337–341
Borer ET, Seabloom EW, Shurin JB, Anderson KE, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2005) What determines the strength of a trophic cascade? Ecology 86:528–537
Borer ET, Halpern BS, Seabloom EW (2006) Asymmetry in community regulation: effects of predators and productivity. Ecology 87:2813–2820
Carpenter SR, Cole JJ, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, Schindler DE (2001) Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol Monogr 71:163–186
Chapman ARO (1986) Population and community ecology of seaweeds. In: Blaxter JHS, Southwood AJ (eds) Advances in marine biology. Academic Press, London
Coley PD, Barone JA (1996) Herbivory and plant defenses in tropical forests. Annu Rev Ecol Syst 27:305–335
Duffy JE, Hay ME (1990) Seaweed adaptations to herbivory—chemical, structural, and morphological defenses are often adjusted to spatial or temporal patterns of attack. Bioscience 40:368–375
Elmhagen B, Rushton SP (2007) Trophic control of mesopredators in terrestrial ecosystems: top–down or bottom–up? Ecol Lett 10:197–206
Eriksson BK, Johansson G (2005) Effects of sedimentation on macroalgae: species-specific responses are related to reproductive traits. Oecologia 143:438–448
Eriksson BK, Rubach A, Hillebrand H (2006) Biotic habitat complexity controls species diversity and nutrient effects on net biomass production. Ecology 87:246–254
Eriksson BK, Rubach A, Hillebrand H (2007) Dominance by a canopy forming seaweed modifies resource and consumer control of bloom-forming macroalgae. Oikos 116:1211–1219
Eriksson BK, Ljunggren L, Sandström A, Johansson G, Mattila J, Rubach A, Råberg S, Snickars M (2009) Declines in predatory fish promote bloom-forming macroalgae. Ecol Appl 19:1975–1988
Estes JA, Tinker MT, Williams TM, Doak DF (1998) Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282:473–476
Fretwell SD (1977) Regulation of plant communities by food-chains exploiting them. Perspect Biol Med 20:169–185
Gruner DS, Smith JE, Seabloom EW, Sandin SA, Ngai JT, Hillebrand H, Harpole WS, Elser JJ, Cleland EE, Bracken MES, Borer ET, Bolker BM (2008) A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol Lett 11:740–755
Heithaus MR, Frid A, Wirsing AJ, Worm B (2008) Predicting ecological consequences of marine top predator declines. Trends Ecol Evol 23:202–210
Herendeen RA (1995) A unified quantitative approach to trophic cascade and bottom–up–top–down hypotheses. J Theor Biol 176:13–26
Herendeen RA (2004) Bottom-up and top-down effects in food chains depend on functional dependence: an explicit framework. Ecol Model 171:21–33
Hopcraft JGC, Olff H, Sinclair ARE (2010) Herbivores, resources and risks: alternating regulation along primary environmental gradients in savannas. Trends Ecol Evol 25:119–128
Ives AR, Cardinale BJ, Snyder WE (2005) A synthesis of subdisciplines: predator-prey interactions, and biodiversity and ecosystem functioning. Ecol Lett 8:102–116
Kiirikki M, Ruuskanen A (1996) How does Fucus vesiculosus survive ice-scraping? Bot Mar 39:133–139
Kraufvelin P, Moy FE, Christie H, Bokn TL (2006) Nutrient addition to experimental rocky shore communities revisited: delayed responses, rapid recovery. Ecosystems 9:1076–1093
Little C, Kitching JA (1996) The biology of rocky shores. Oxford University Press, Oxford
Lotze HK, Worm B (2000) Variable and complementary effects of herbivores on different life stages of bloom-forming macroalgae. Mar Ecol Prog Ser 200:167–175
Lotze HK, Worm B, Sommer U (2000) Propagule banks, herbivory and nutrient supply control population development and dominance patterns in macroalgal blooms. Oikos 89:46–58
McLaren BE, Peterson RO (1994) Wolves, moose, and tree-rings on Isle Royale. Science 266:1555–1558
Menge BA (2000) Top-down and bottom-up community regulation in marine rocky intertidal habitats. J Exp Mar Biol Ecol 250:257–289
Moksnes P-O, Gullstrom M, Tryman K, Baden S (2008) Trophic cascades in a temperate seagrass community. Oikos 117:763–777
Nisbet RM, Diehl S, Wilson WG, Cooper SD, Donalson DD, Kratz K (1997) Primary-productivity gradients and short-term population dynamics in open systems. Ecol Monogr 67:535–553
Oksanen L, Oksanen T (2000) The logic and realism of the hypothesis of exploitation ecosystems. Am Nat 155:703–723
Oksanen L, Fretwell SD, Arruda J, Niemela P (1981) Exploitation ecosystems in gradients of primary productivity. Am Nat 118:240–261
Oksanen T, Power ME, Oksanen L (1995) Ideal free habitat selection and consumer-resource dynamics. Am Nat 146:565–585
Olff H, Alonso DA, Berg MP, Eriksson BK, Loreau M, Piersma T, Rooney N (2009) Parallel interaction webs in ecosystems. Philos Trans Royal Soc B 364:1755–1779
Persson A, Hansson LA, Bronmark C, Lundberg P, Pettersson LB, Greenberg L, Nilsson PA, Nystrom P, Romare P, Tranvik L (2001) Effects of enrichment on simple aquatic food webs. Am Nat 157:654–669
Polis GA (1999) Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86:3–15
Polis GA, Strong DR (1996) Food web complexity and community dynamics. Am Nat 147:813–846
Polis GA, Sears ALW, Huxel GR, Strong DR, Maron J (2000) When is a trophic cascade a trophic cascade? Trends Ecol Evol 15:473–475
Rangeley RW, Thomas MLH (1987) Predatory behaviour of juvenile shore crab Carcinus maenas (L). J Exp Mar Biol Ecol 108:191–197
Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2002) A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:785–791
Sieben K, Rippen AD, Eriksson BK (2011) Cascading effects from predator removal depend on resource availability in a benthic food web. Mar Biol 158:391–400
Sinclair ARE, Krebs CJ, Fryxell JM, Turkington R, Boutin S, Boonstra R, Seccombe-Hett P, Lundberg P, Oksanen L (2000) Testing hypotheses of trophic level interactions: a boreal forest ecosystem. Oikos 89:313–328
Strong DR (1992) Are trophic cascades all wet? Differentiation and donor-control in speciose ecosystems. Ecology 73:747–754
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. In: Johnston RF (ed) Annual review of ecology and systematics. Blackwell Scientific, Palo Alto
Winemiller KO (1989) Ontogenetic diet shifts and resource partitioning among piscivorous fishes in the Venezuelan Llanos. Environ Biol Fish 26:177–199
Wootton JT, Power ME (1993) Productivity, consumers, and the structure of a river food-chain. Proc Natl Acad Sci USA 90:1384–1387
Worm B, Lotze HK (2006) Effects of eutrophication, grazing, and algal blooms on rocky shores. Limnol Oceanogr 51:569–579
Worm B, Reusch TBH, Lotze HK (2000) In situ nutrient enrichment: methods for marine benthic ecology. Int Rev Hydrobiol 85:359–375
Worm B, Lotze HK, Sommer U (2001) Algal propagule banks modify competition, consumer and resource control on Baltic rocky shores. Oecologia 128:281–293
Acknowledgments
This study was supported by the Swedish Research Council for the Environment, Agricultural Sciences and Spatial Planning (Formas 223-2005-1187). We thank Jim Coyer, Tjisse van der Heide, and two anonymous reviewers for helpful comments on the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Eriksson, B.K., Rubach, A., Batsleer, J. et al. Cascading predator control interacts with productivity to determine the trophic level of biomass accumulation in a benthic food web. Ecol Res 27, 203–210 (2012). https://doi.org/10.1007/s11284-011-0889-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-011-0889-1