Abstract
The sheep is one of the most successful and widely spread domestic animals. Archaeological evidence traces the first domestic sheep back to the Near East region around 9,000 years ago. It is also known that soon after, the domesticated sheep started to flow out of the centre of origin and spread all over the ancient world following the expansion of agriculture. Throughout time, herders, nature elements and eventually some hybridization with different wild relatives produced a multitude of breeds. However, until the advent of the molecular genetics field, very little was known about the origins of most of those breeds. Two decades after the first genetic studies, we have gathered considerable information on the origins, phylogenetic relationships and patterns of genetic diversity of the sheep across the world. Indeed, the genetic studies confirmed the Near East region as the main centre of origin and also revealed other contributions from other regions. Specifically about the fat-tailed sheep, molecular genetics was also able to link their maternal origin to a specific group. So far, modern sheep have originated from five different maternal origins. Nonetheless, the technological advances of the DNA sequencing techniques are bringing more data that is showing the complexity of the domestication process.
Similar content being viewed by others
Introduction
Domestication of sheep (Ovis aries) occurred in the Fertile Crescent in the Neolithic period, constituting a shift from food acquisition to food production, about 9,000 years ago (see Legge 1996). Human-mediated breeding has subsequently generated specialized breeds suitable for a diverse range of purposes including the production of wool, meat and milk. Since domestication, sheep have established a wide geographic range due to their adaptability to nutrient-poor diets, tolerance to extreme climatic conditions and their manageable size (Kijas et al. 2009). Curiously, in contrast with other regions, the pastoral management of cattle, sheep and goats was widespread in Africa thousands of years before the settled agricultural communities or the use of domesticated plants (Marshall 1994; Marshall 2000).
Here, we will present a digest of the most recent findings on the origins and spread of fat-tailed sheep. Because this variety of sheep shares a common history with other varieties, we will therefore start by explaining the origins and domestication of the sheep and later introduce what is known about the fat-tailed variant. It is worth noting that the studies aiming to assess the genetic origins and relationships of fat-tailed sheep are the product of a broad range of scientific areas, such as molecular genetics, taxonomy, archaeology and anthropology.
The wild ancestor and origin of domestic sheep
Despite the several genetic studies attempting to unveil the exact wild ancestor of the domestic sheep, it still remains unclear (Meadows et al. 2007; Meadows et al. 2011). Several species or subspecies of the genus Ovis have been suggested to be the wild ancestor of domestic sheep. The genus Ovis had an Asiatic origin and migrated to North America through North-Eastern Asia and the Bering Strait; diversified in Eurasia less than three million years ago resulting in successive speciation events occurring along the migration routes (Rezaei et al. 2010). Last update of the taxonomic species nomenclature divided the genus Ovis into seven species (Festa-Bianchet 2000), from which the Asiatic mouflon (Ovis orientalis) is considered the most probable ancestor of all domestic sheep (Hiendleder et al. 2002; Bruford and Townsend 2006; Rezaei et al. 2010).
Archaeological evidence shows the oldest domestication centres of sheep and goat to have occurred in the Neolithic period, 9,000–8,000 years ago, in the Fertile Crescent where many settlements have been discovered (see Legge 1996; Meadows et al. 2007). Sheep were among the first livestock species to be domesticated (Ryder 1984), and it is supposed that in the early times, domesticated sheep were selected for their meat and only later started to be selected for secondary products, such as wool. The number of culturally and biologically independent domestication events may be lower than the number of distinct lineages because the original wild populations may have been polymorphic, or new maternal lineages may have been introgressed from different wild populations into the domesticated populations (Zeder et al. 2006).
One of the ancient findings of sheep bones in a pre-pottery Neolithic archaeological site, was found in Jericho and dates back to a period between 9,000–10,000 year before present (BP) (Clutton-Brock and Uerpmann 1974). But the earliest dated site to produce remains of sheep that are assumed to have been under human control, if not actually domesticated, is Zawi Chemi Shanidar in northeast Iran. This site has a radiocarbon date of 10,870 ± 300 bp, which makes it rather older than that of Jericho from which two sheep bones have been retrieved. The remains of sheep from these early sites do not show morphological changes that indicate the influence of man. There is little doubt that it was during the eighth and seventh millennia bc that man first began to domesticate sheep within the region of western Asia and that these early pastoralists spread rather rapidly westwards into Europe and probably north and east into Asia and the Far-East (Clutton-Brock 1999).
Morphological changes that were observed during the analysis of the sheep remains from later Neolithic sites (sixth and fifth millennia before Christ (bc)) are the absence of horns in the females and a shortening of the limb bones. It is probable that the fleece changed from the wild type as a result of mutations followed by artificial selection, perhaps as early as the sixth millennium bc. The outer coat of wild sheep is stiff and hairy and covers a short woolly undercoat which only grows in the winter. In highly domesticated sheep, kemps are absent and the fleece consists entirely of woolly undercoat which grows all year round and is not shed in the summer (Clutton-Brock 1999). Most of the features of domestication in the sheep, such as alteration of horn shape, hornless ewes, fattiness and length of the tail, and the woolly, white fleece, were already common in western Asia by 3000 bc. For all these characters are shown in pictorial representation in Mesopotamia and are also written about in the Babylonian texts. Indeed, changes in body size is frequently a marker of domestication (Davis and Beckett 1999).
As far as the fat-tailed sheep in Africa is concerned, Egypt witnessed the arrival of screw-horned sheep during the Early Dynastic Period (3100–2613 bc) and fat-tailed sheep in the Middle Kingdom between 1991 and 1633 bc (Zeuner 1963; Ryder 1983; Clutton-Brock 1993). Sheep appear in the East African archaeological record by about 4500 bp (Phillipson 1977; Barthelme 1985; Marshall 1994). South Africa saw the arrival of domestic sheep by about 2000 bp (Schweitzer and Scott 1973; Sealy and Yates 1994; Henshilwood 1996; Webley 2001). Rock art depictions of both fat-tailed and thin-tailed sheep have been reported from Zimbabwe (Goodall 1946), and of fat-tailed sheep in southern Natal (Vinnicombe 1976) and at the Cape (Robertshaw 1978, Manhire et al. 1984, Manhire et al. 1986; Jerardino 1999).
The possible origin of the fat-tailed sheep
It is generally accepted that main changes in morphological traits such as the lengthening of the tail and distinct patterns of fat accumulation in the tail are considered to be some of the major changes that followed domestication. The earliest fat-tailed animals represented had a short and broad tail and selection of this trait was towards an increase of fat (Ryder 1991).
The fat deposits on the tail represent an accumulation of reserve material, similar to the humps of the camel and dromedary (Epstein 1985). Such deposits were evolved under steppe and desert conditions, noted for long periods of drought and scarcity of feed. The fat-tail points, therefore, to a steppe country in central Asia/Mesopotamia, where several rock engravings of fat-tailed sheep with spears pointing at their bodies, are depicted as the origin of evolution of these sheep. In the steppe and desert countries and among peoples lacking other fat-producing animals, fat tails that sporadically occurred in sheep were considered to be important, and thereafter, sheep with adipose deposits in the tail were particularly selected for breeding purposes (Epstein 1985). The fat tail may have been acquired long after the domestication of the long and thin-tailed breeds of Asia. Hence, the ancestral wild stock of both thin- and fat-tailed sheep is identical (Epstein 1971).
Insights into origin and domestication of domestic sheep from molecular markers
Molecular marker, also known as genetic marker, is a gene or DNA sequence with a known location in the genome of an organism at which the DNA base sequence varies among different individuals of a population or species. Such markers have been widely used for a variety of purposes, including chromosome mapping, DNA fingerprinting and genetic screening. In addition, genetic markers have been applied to estimate evolutionary rates, processes and constraints on molecular evolution or to infer population history and species phylogeny.
Genetic markers are generally classified into several types, including restriction fragment length polymorphisms, variable number tandem repeats, microsatellite DNA and single nucleotide polymorphisms (SNPs). Based on features of inheritance, genetic markers can be categorized as maternal (mitochondrial DNA—mtDNA), paternal (Y chromosome), bi-parental or autosomal. mtDNA and Y chromosome markers provide phylogenetic history of maternal and paternal lineages, and identify female- or male-mediated genetic introgression, respectively. They can be used to pinpoint geographic centre of origin of domestic species or to infer population history and migration route. Autosomal markers reveal complex history from both maternal and paternal inheritance. In addition, autosomal markers can provide information about genome-wide diversity and bi-directional genetic introgression.
Maternally inherited mitochondrial DNA
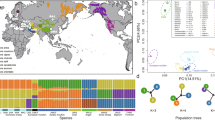
In the advent of the PCR, back in the 1990s of the last century, the first studies on domestic sheep could only detect two domestic mtDNA lineages (Hiendleder et al. 1998; Hiendleder et al. 2002). But soon after, the enlargement of the sample size covering more geographical regions, resulted in the identification of five different mtDNA lineages (Fig. 1; Meadows et al. 2007), indicating that modern sheep resulted from multiple domestication events (Pedrosa et al. 2005; Tapio et al. 2006; Meadows et al. 2007; Chessa et al. 2009). Very recently, the complete mitochondrial genomes (mitogenomes) from representatives of each of the five mtDNA lineages (Fig. 1) were analyzed and revealed to have radiated from a common ancestor around 920,000 ± 190,000 years ago (Meadows et al. 2011) (Fig. 2).
Unrooted neighbour-joining consensus-tree depicting the relationships between 57 European, African and Middle-Eastern sheep breeds. Genetic distances were based on 28 microsatellite markers. For breed abbreviations and further methodological details see Peter et al. (2007). Reproduced, with permission, from Peter et al. (2007)
Theoretically, the presence of various maternal lineages may be interpreted as originating from a single large population containing highly divergent lineages but it is more likely that they originated from several smaller wild populations (Pedrosa et al. 2005). Since the beginning of the last century, it has been postulated that populations from centres of domestication (i.e., place in which domestication took place) tend to retain larger genetic diversity (Vavilov 1992). In the last years, several studies using mtDNA sequencing to estimate genetic diversity, support the Fertile Crescent as the origin of domestication for four of those mtDNA lineages (A, B, C, and D), and Caucasus as the probable domestication centre of lineage E. Remarkably, lineage C has been observed mostly in fat-tail sheep breeds (Pedrosa et al. 2005; Tapio et al. 2006; Meadows et al. 2007).
Concerning the geographic distribution of these five mtDNA lineages, the A and B are the most widespread and frequently distributed lineages. These variants were first documented by Wood and Phua (1996) and classified by Hiendleder et al. (1998), but have since been found in every geographic region where domestic sheep have been sampled (Meadows et al. 2011). Regarding the phylogenetic origins of these two mtDNA lineages, Tapio et al. (2006) have shown that they derived from the wild populations approximately at the same time (though B is more recent than A) in the Near East, making their fully independent derivation from wild sheep unlikely. Lineage A shows no close relationship with any of the current wild sheep species investigated, but nonetheless, Hiendleder et al. (2002) and Pedrosa et al. (2005) proposed the Asiatic mouflon populations (O. orientalis) inhabiting the region known today as Iran, as its most probable ancestor species. Lineage B, the European lineage, predominates in the European domestic sheep and in the feral European mouflon (Ovis musimon) (Hiendleder et al. 2002). However, it is important to mention that the European mouflon can represent a primitive domesticate of the Near Eastern mouflon (O. orientalis), that was transported into Europe from Asia after the Neolithic period (Meadows et al. 2007).
The third mtDNA lineage, lineage C, was observed for the first time in Chinese and Turkish sheep breeds (Guo et al. 2005; Pedrosa et al. 2005; Chen et al. 2006). Population genetic analyses showed that this lineage was the most recent mtDNA lineage to have expanded (Tapio et al. 2006) and was only observed in Asia (Meadows et al. 2007) with the exception of Portuguese sheep, in which lineage C has been reported at a low frequency (Pereira et al. 2006). This lineage has been mainly found in the fat-tailed breeds and its distribution overlays with that of the fat-tailed sheep, namely, semi-desert and steppe regions around the Caspian Sea, Central Asia and China (Ryder 1984), suggesting lineage C to be associated with the fat-tailed sheep (Tapio et al. 2006).
The mtDNA lineages D and E were the last ones to be observed in individuals from the Near East (Tapio et al. 2006; Meadows et al. 2007). Lineages D and E, are also the rarest, only being found in sheep from the Caucasus and Turkey (Tapio et al. 2006; Meadows et al. 2007; Meadows et al. 2011). Lineage D was discovered from a single sheep sampled from the north Caucasus, which grouped separately from the three defined ovine mitochondrial DNA (mtDNA) clades (Tapio et al. 2006). Although this lineage grouped with wild Ovis species, it did not form a distinct cluster within the Asiatic mouflon (O. orientalis), urial (O. vignei) or argali (O. ammon). Interestingly, although rare in domestic animals, such haplotypes of lineages C, D and E may represent a direct link with their wild ancestors through introgression from wild sheep populations, even with the discovery of these new clades, no extant wild Ovis progenitor has been identified (Horsburgh and Rhines 2010; Meadows et al. 2011).
Autosomal markers—microsatellites and SNPs
Microsatellites
The natural history of domestic sheep cannot be assessed without having information from the nuclear genome. However, the number of studies involving transcontinental sampling and using nuclear genomic markers is far smaller than those using mitochondrial genome. From these few studies there are at least three that are worthy of reference here.
To understand the genetic diversity and genetic relationships between Turkish sheep breeds, Uzun et al. (2006) used a set of 30 microsatellite markers. Interestingly, they found that Akkaraman and Morkaraman breeds, both fat-tailed sheep, were found to have remarkable close genetic relationships, despite the fact that the former is found in Central Anatolia and is mainly selected for meat production, and the latter is only found in Eastern Anatolia and is selected for coarse wool. Moreover, they could clearly identify a genetic separation between fat-tailed sheep and the other types of Turkish breeds. Peter et al. (2007) used a set of 31 microsatellites to analyze 57 sheep breeds from Europe, Near East and Arabic Peninsula and also found that fat-tailed sheep grouped into a separate genetic cluster.
In another microsatellite analysis study on local sheep breeds from North Eurasia (Caucasus, Asia and the eastern fringe of Europe, including central and western Russia), three distinct genetic groups were identified, from which one is constituted of fat-tailed sheep breeds (Tapio et al. 2010). The fat-tailed sheep used in this study had coarse wool and are native to the Caucasus and Caspian basin areas. The fat-tailed cluster was further divided into Andi (from mountains) and Karakul (steppe-desert) type sheep, which showed a varying degree of admixture between them. However, the majority of fat-tailed breeds have their ancestries in the first two subclusters (Andi and Karakul types) showing substantial gene flow between them (Tapio et al. 2010). Highest genetic diversity was found in the southern periphery of the studied area (Caspian Sea and Black Sea basins), supporting this region as the place of origin for fat-tailed sheep.
Finally, as the samples used in Uzun et al. (2006) were also analyzed for their mtDNA (Pedrosa et al. 2005), the authors of this study compared the results of both markers. It is reported that the fat-tailed sheep do not share similar mtDNA lineages. Akkaraman presents type C mtDNA, while the closely related Morkaraman breed presents the Asian lineage (type A mtDNA) which is in accordance with its location in Eastern Turkey and Western Iran; therefore, the close relationship detected between these two breeds were apparently not obtained through the maternal lineages.
Autosomal SNPs
The genomic abundance, the reduced cost and the effective high-throughput genotyping make the SNPs as the most promising class of genetic marker in population genetic studies. The first genome-wide set of SNP markers (autosomal variation on a global scale) for sheep was recently published (Kijas et al. 2009) and did not report any particular analysis in what concerns genetic relationships between and within different fat- and thin-tailed or any other group of sheep breeds. However, cluster analysis and the partitioning of SNP variation revealed that sheep have weak phylogeographic structure. The finding that only 5.8% of variation was partitioned between geographic groupings and 82.2% was resident within breeds indicates that sheep have the weakest phylogeographic structure of any domestic species examined to date (Kijas et al. 2009). Nonetheless, despite the low degree of population substructuring, it was still possible to assign individuals to its geographic origin.
Y chromosome SNPs and microsatellites
The mammalian Y chromosome has two components, a pseudo-autosomal region, which frequently recombines with the X chromosome, and a male-specific region (MSY). Little is known about the ovine MSY. Each of the six markers which form the linkage map of the ovine Y chromosome is located within the pseudo-autosomal region and is not male specific (Maddox et al. 2001). So far, sequence data is available for only two Y chromosome genes: SRY and ZFY (Payen et al. 1996; Lawson and Hewitt 2002), but no informative polymorphic sites were found in O. aries populations. There are also a small number of known genes within the MSY region and there is almost lack of genetic variation in few ovine MSY sequences from public databases. This is not surprising since it is known that nucleotide diversity in the mammalian Y is generally lower than that found on autosomes (Hellborg and Ellegren 2004).
Analysis of a set of SNPs and microsatellites from the MRY region has revealed patterns of male-mediated introgression during breed differentiation (Meadows et al. 2004; Meadows et al. 2006), but those studies did not test for differentiation between fat-tailed sheep and the other groups of sheep. It is likely, however, that the low level of nucleotide diversity observed in the ovine Y would not be able to differentiate between those sheep groups. Notwithstanding, a recent study on Turkish sheep showed a different distribution for three alleles at SRYM18 locus between thin- and fat-tailed sheep breeds, of which the 145-bp allele was exclusively detected at low frequency in the fat-tailed sheep (Oner et al. 2010).
Discussion
From the small amount of genetic studies done on domestic sheep, it was only possible to extract limited information on the origins and genetic relationships of the fat-tailed sheep. Genetic data indicates that the most probable centre of origin was the Caucasus region and that fat-tailed sheep have high degree of genetic similarity that is not supported by a common maternal origin.
Although the cultural and economical interest in the fat-tailed sheep is well documented, there are currently no publications reporting genetic studies aiming to improve the knowledge on the origins of this type of sheep, nor are there publications reporting the average genetic diversity of the fat-tailed sheep breeds, how much they contribute to the total sheep gene pool, and their genetic significance and distinctiveness. Morphological and behavioural characteristics, along with chromosome number, are not enough to infer evolutionary history, nor classification of the Ovis species. Therefore, further molecular data analyses are essential to unravel the mysteries of relationships within species of the Ovis genus.
However, the arrival of next-generation DNA sequencing technologies have made available a growing number of SNP sets that for sure will shed new light on the genetic origins of the fat-tailed sheep.
References
Barthelme, J. W., 1985. Fisher-hunters and neolithic pastoralists in East Turkana, Kenya, (British Archaeological Reports, Oxford)
Bruford, M. W. and Townsend, S. J., 2006. Mitochondrial DNA diversity in modern sheep. In: M. A. Zeder, et al. (eds), Documenting domestication: new genetic and archaeological paradigms, (University of California Press, Berkeley), 306–316
Chen, S.-Y., Duanb, Z.-Y., Shaa, T., Xiangyub, J., Wub, S.-F. and Zhang, Y.-P., 2006. Origin, genetic diversity, and population structure of Chinese domestic sheep, Gene, 376, 216–223
Chessa, B., Pereira, F. and Arnaud, F., 2009. Revealing the history of sheep domestication using retrovirus integrations, Science, 324, 532–536
Clutton Brock, J. and Uerpmann, H.-P., 1974. The sheep of early Jericho, Journal ofArchaeological Science, 1, 261–274
Clutton-Brock, J., 1993. The spread of domestic animals in Africa. In: T. Shaw, et al. (eds), The Archaeology of Africa: Food, Metals and Towns, (Routledge, New York), 61–70
Clutton-Brock, J., 1999. A Natural History of Domesticated Mammals, (Cambridge University Press, Cambridge)
Davis, S. J. M. and Beckett, J. V., 1999. Animal husbandry and agricultural improvement: The archaeological evidence from animal bones and teeth, rural history, 10, 1–17
Epstein, H., 1971. The origin of the domestic animals of Africa, (Africana Publishing Corporation, New York)
Epstein, H., 1985. The Awassi sheep with special reference to the improved dairy type, (FAO, Rome)
Festa-Bianchet, M., 2000. A summary of discussion on the taxonomy of mountain ungulates and its conservation implications, Workshop on Caprinae taxonomy (Ankara, Turkey)
Goodall, E., 1946. Domestic animals in rock art, Transactions of the Rhodesia Scientific Association, 41, 57–62
Guo, J., Du, L. X., Ma, Y. H., Guan, W. J., Li, H. B., Zhao, Q. J., Li, X. and Rao, S. Q., 2005. A novel maternal lineage revealed in sheep (Ovis aries), Animal Genetics, 36, 331–336
Hellborg, L. and Ellegren, H., 2004. Low levels of nucleotide diversity in mammalian Y chromosomes, Molecular Biology and Evolution, 21, 158–163
Henshilwood, C., 1996. A revised chronology for pastoralism in southernmost Africa: new evidence of sheep at c. 2000 b.p. from Blombos Cave, South Africa, Antiquity, 70, 945–949
Hiendleder, S., Mainz, K., Plante, Y. and Lewalski, H., 1998. Analysis of mitochondrial DNA indicates that domestic sheep are derived from two different ancestral maternal sources: no evidence for contributions from urial and argali sheep, Journal of Heredity, 89, 113–120
Hiendleder, S., Kaupe, B., Wassmuth, R. and Janke, A., 2002. Molecular analysis of wild and domestic sheep questions current nomenclature and provides evidence for domestication from two different subspecies, Proceedings of the Royal Society B: Biological Sciences, 269, 893–904
Horsburgh, K. A. and Rhines, A., 2010. Genetic characterization of an archaeological sheep assemblage from South Africa's Western Cape, Journal of Archaeological Science, 37, 2906–2910
Jerardino, A., 1999. A first account of fat-tailed sheep in the rock paintings of the Western Cape coast, South African Archaeological Bulletin, 54, 64–66
Kijas, J., Townley, D. and Dalrymple, B., 2009. A genome wide survey of SNP variation reveals the genetic structure of sheep breeds, PloS one, 4, e4668
Lawson, L. J. and Hewitt, G. M., 2002. Comparison of substitution rates in ZFX and ZFY introns of sheep and goat related species supports the hypothesis of male-biased mutation rates, Journal of Molecular Evolution, 54, 54–61
Legge, T., 1996. The beginnings of caprine domestication. In: D. R. Harris (eds), The Origins and Spread of Agriculture and Pastoralism in Eurasia, (Smithsonian Institution Press, New York), 238–262
Maddox, J. F., Davies, K. P. and Crawford, A. M., 2001. An enhanced linkage map of the sheep genome comprising more than 1000 loci, Genome Research, 11, 1275–1289
Manhire, A. H., Parkington, J. E. and Robey, T. S., 1984. Stone tools and Sandveld settlement. In: M. Hall, et al. (eds), Frontiers: Southern African Archaeology Today, (BAR International Series, Cambridge), 111–120
Manhire, A. H., Parkington, J. E., Mazel, A. D. and Maggs, T. M. O. C., 1986. Cattle, sheep and horses: a review of domestic animals in the rock art of southern Africa, South African Archaeological Bulletin (Goodwin Series), 5, 22–30
Marshall, F., 1994. Archaeological Perspectives on East African pastoralism. In: E. Fratkin, et al. (eds), African Pastoralist Systems: An Integrated Approach, (Lynne Rienner Publishers, Boulder, CO), 17–43
Marshall, F., 2000. The origins and spread of domestic animals in East Africa. In: R. M. Blench and MacDonald, K. C. (eds), The origin and development of African livestock, (University College Press., London), 191–221
Meadows, J. R. S., Hawken, R. J. and Kijas, J. W., 2004. Nucleotide diversity on the ovine Y chromosome, Animal Genetics, 35, 379–385
Meadows, J. R. S., Hanotte, O., Drögemüller, C., Calvo, J., Godfrey, R., Coltman, D., Maddox, J. F., Marzanov, N., Kantanen, J. and Kijas, J. W., 2006. Globally dispersed Y chromosomal haplotypes in wild and domestic sheep, Animal Genetics, 37, 444–453
Meadows, J. R. S., Cemal, I., Karaca, O., Gootwine, E. and Kijas, J. W., 2007. Five ovine mitochondrial lineages identified from sheep breeds of the near east, Genetics, 175, 1371–1379
Meadows, J. R. S., Hiendleder, S. and Kijas, J. W., 2011. Haplogroup relationships between domestic and wild sheep resolved using a mitogenome panel, Heredity, 06:700-706. doi:10.1038/hdy.2010.122.
Oner, Y., Calvo, J. H. and Elmaci, C., 2010. Y chromosomal characterization of Turkish native sheep breeds, Livestock Science, doi:10.1016/j.livsci.2010.08.015
Payen, E., Pailhoux, E., Abou Merhi, R., Gianquinto, L., Kirszenbaum, M., Locatelli, A. and Cotinot, C., 1996. Characterization of ovine SRY transcript and developmental expression of genes involved in sexual differentiation, International Journal of Developmental Biology, 40, 567–575
Pedrosa, S., Uzun, M., Arranz, J.-J., Gutiérrez-Gil, B., San Primitivo, F. and Bayón, Y., 2005. Evidence of three maternal lineages in near eastern sheep supporting multiple domestication events, Proceedings of the Royal Society of London B, 272, 2211–2217
Pereira, F., Davis, S. J. M., Pereira, L., McEvoy, B., Bradley, D. G. and Amorim, A., 2006. Genetic signatures of a Mediterranean influence in Iberian Peninsula sheep husbandry, Molecular Biology and Evolution, 23, 1420–1426
Peter, C., Bruford, M. and Perez, T., 2007. Genetic diversity and subdivision of 57 European and Middle-Eastern sheep breeds, Animal Genetics, 38, 37–44
Phillipson, D. W., 1977. The Later Prehistory of Eastern and Southern Africa, (Heinemann, London)
Rezaei, H. R., Naderi, S., Chintauan-Marquier, I. C., Taberlet, P., Virk, A. T., Naghash, H. R., Rioux, D., Kaboli, M. and Pompanon, F., 2010. Evolution and taxonomy of the wild species of the genus Ovis (Mammalia, Artiodactyla, Bovidae), Molecular Phylogenetics and Evolution, 54, 315–326
Robertshaw, P. T., 1978. The origin of pastoralism in the Cape, South African Historical Journal, 10, 117–133
Ryder, M., 1991. Domestication, history and breed evolution in sheep, (Elsevier, Amsterdam)
Ryder, M. L., 1983. Sheep and Man, (Duckworth, London)
Ryder, M. L., 1984. Sheep. In: I. L. Mason (eds), Evolution of domesticated animals, (Longman, London), 63–85
Schweitzer, F. R. and Scott, K. J., 1973. Early occurrence of domestic sheep in sub-Saharan Africa, Nature, 241, 547
Sealy, J. and Yates, R., 1994. The chronology of the introduction of pastoralism to the Cape, South Africa, Antiquity, 68, 58–67
Tapio, M., Marzanov, N., Ozerov, M., Ćinkulov, M., Gonzarenko, G., Kiselyova, T., Murawski, M., Viinalass, H. and Kantanen, J., 2006. Sheep Mitochondrial DNA Variation in European, Caucasian, and Central Asian Areas, Molecular Biology and Evolution, 23, 1776–1783
Tapio, M., Ozerov, M., Tapio, I., Toro, M., Marzanov, N., Cinkulov, M., Goncharenko, G., Kiselyova, T., Murawski, M. and Kantanen, J., 2010. Microsatellite-based genetic diversity and population structure of domestic sheep in northern Eurasia, BMC Genetics, 11, 76
Uzun, M., Gutiérrez-Gil, B., Arranz, J.-J., San Primitivo, F., Saatci, M., Kaya, M. and Bayón, Y., 2006. Genetic relationships among Turkish sheep, Genetics, Selection and Evolution, 38, 513–524
Vavilov, N., 1992. Origin and Geography of Cultivated Plants, (Cambridge University Press, Cambridge)
Vinnicombe, P., 1976. People of the Eland: Rock Paintings of the Drakensberg Bushmen as a Reflection of their Life and Thought, (University of Natal Press, Pietermaritzburg, South Africa)
Webley, L., 2001. The re-excavation of Spoegrivier Cave on the west coast of southern Africa, Annals of the Eastern Cape Museums, 2, 19–49
Wood, N. J. and Phua, S. H., 1996. Variation in the control region sequence of the sheep mitochondrial genome, Animal Genetics, 27, 25–33
Zeder, M., Emshwiller, E., Smith, B. D. and Bradley, D. G., 2006. Documenting domestication: the intersection of genetics and agriculture, Trends in Genetics, 22, 139–155
Zeuner, F. E., 1963. A History of Domesticated Animals, (Hutchinson, London)
Acknowledgements
SC is the recipient of a FCT individual fellowship grant SFRH/BPD/46082/2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocha, J., Chen, S. & Beja-Pereira, A. Molecular evidence for fat-tailed sheep domestication. Trop Anim Health Prod 43, 1237–1243 (2011). https://doi.org/10.1007/s11250-011-9854-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-011-9854-9