Abstract
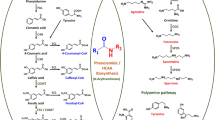
Gamma linolenic acid (GLA; C18:3Δ6,9,12 cis), also known as γ-Linolenic acid, is an important essential fatty acid precursor for the synthesis of very long chain polyunsaturated fatty acids and important pathways involved in human health. GLA is synthesized from linoleic acid (LA; C18:2Δ9,12 cis) by endoplasmic reticulum associated Δ6-desaturase activity. Currently sources of GLA are limited to a small number of plant species with poor agronomic properties, and therefore an economical and abundant commercial source of GLA in an existing crop is highly desirable. To this end, the seed oil of a high LA cultivated species of safflower (Carthamus tinctorius) was modified by transformation with Δ6-desaturase from Saprolegnia diclina resulting in levels exceeding 70% (v/v) of GLA. Levels around 50% (v/v) of GLA in seed oil was achieved when Δ12-/Δ6-desaturases from Mortierella alpina was over-expressed in safflower cultivars with either a high LA or high oleic (OA; C18:1Δ9 cis) background. The differences in the overall levels of GLA suggest the accumulation of the novel fatty acid was not limited by a lack of incorporation into the triacylgylcerol backbone (>66% GLA achieved), or correlated with gene dosage (GLA levels independent of gene copy number), but rather reflected the differences in Δ6-desaturase activity from the two sources. To date, these represent the highest accumulation levels of a newly introduced fatty acid in a transgenic crop. Events from these studies have been propagated and recently received FDA approval for commercialization as Sonova™400.



Similar content being viewed by others
References
Abbadi A, Domergue F, Bauer J, Napier JA, Welti R, Zähringer U, Cirpus P, Heinz E (2004) Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16:2734–2748
Badami RC, Patil KB (1980) Structure and occurrence of unusual fatty acids in minor seed oils. Prog Lipid Res 19:119–153
Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, Murray E, Morck D, Moloney MM (2010) Seed-based expression systems for plant molecular farming. Plant Biotechnol J 8:588–606
Brenner RR (1976) Regulatory function of Δ6-desaturase-a key enzyme of polyunsaturated fatty acid synthesis. Adv Exp Med Biol 83:85–101
Cahoon EB, Ripp KG, Hall SE, McGonigle B (2002) Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiol 128:615–624
Cheng B, Wu G, Vrinten P, Falk K, Bauer J, Qiu X (2010) Towards the production of high levels of eicosapentaenoic acid in transgenic plants: the effects of different host species, genes and promoters. Transgenic Res 19:221–229
Dahlqvist A, Ståhl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Ad Stymne S (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97:6487–6492
Damude HG, Kinney AJ (2007) Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids 42:179–185
Das UN (1990) Gamma-linolenic acid, arachidonic acid, and eicosapentaenoic acid as potential anticancer drugs. Nutrition 6:429–434
Domergue F, Lerchl J, Zahringer U, Heinz E (2002) Cloning and functional characterization of Phaeodactylum tricornutum front-end desaturases involved in eicosapentaenoic acid biosynthesis. Eur J Biochem 269:4105–4113
Ekin Z (2005) Resurgence of safflower (Carthamus tinctorius L.) utilization: a global view. J Agron 4:83–87
Fidantsef A, van Boxtel J, Faccioti D, Gadisman M, Goodstal JJ, Graham K, Harry I, Moloney M, Ng W, Nykiforuk C, Sanchez I, Villa N, Williams K (2008) Transgenic production of high GLA safflower (Carthamus tinctorius L.) oil. 18th international conference on plant lipids, Bordeaux, France
Flider FJ (2005) GLA: uses and new sources. Inform 16:279–282
Garcia-Maroto F, Garrido-Cardenas JA, Rodriguez-Ruiz J, Vilches-Ferron M, Adam AC, Polaina J, Alonso DL (2002) Cloning and molecular characterization of the Δ6-desaturase from two Echium plant species: production of GLA by heterologous expression in yeast and tobacco. Lipids 37:417–426
Gill I, Valivety R (1997) Polyunsaturated fatty acids, part 1: occurrence, biological activities and applications. Trends Biotechnol 15:401–409
Griffin BA (2008) How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr Opin Lipidol 19:57–62
Hibbeln JR, Nieminen LRG, Lands WEM (2004) Increasing homicide rates and linoleic acid consumption among five western countries. Lipids 39:1207–1213
Hibbeln JR, Nieminen LRG, Blasbalg TL, Riggs JA, Lands WEM (2006) Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr 83(suppl):1483S–1493S
Hong H, Datla N, Reed DW, Covello PS, MacKenzie SL, Qiu X (2002) High-level production of γ-linolenic acid in Brassica juncea using a Δ6 desaturase from Pythium irregulare. Plant Physiol 129:354–362
Horrobin DF (1992) Nutritional and medical importance of gamma-linolenic acid. Prog Lipid Res 31:163–194
Huang Y-S, Chaudhary S, Thurmond JM, Bobik EG, Yuan L, Chan GM, Kirchner SJ, Mukerji P, Knutzon DS (1999) Cloning of Δ12- and Δ6-desaturases from Mortierella alpina and recombinant production of γ-linolenic acid in Saccharomyces cerevisiae. Lipids 34:649–659
Hudson BJ (1984) Evening primrose (Oenothera spp.) oil and seed. J Am Oil Chem Soc 61:540–542
Karlstad MD, Palombo JD, Murray MJ, DeMichele SJ (1996) The anti-inflammatory role of γ-linolenic and eicosapentaenoic acids in acute lung injury. In: Huang Y-S, Mills DE (eds) γ-Linolenic acid, metabolism and its roles in nutrition and medicine. AOCS Press, pp 37–167
Kinney AJ, Cahoon EB, Hitz WD (2002) Manipulating desaturase activities in transgenic crop plants. Biochem Soc Trans 30:1099–1103
Kinney AJ, Cahoon EB, Damude HG, Hitz WD, Kolar CW, Liu ZB (2004) Production of very long chain polyunsaturated fatty acids in oilseed plants. PCT International Application WO/2004/4071467
Knauf VC, Shewmaker C, Flider F, Emlay D, Rey E (2011) Safflower with elevated gamma linolenic acid. US Patent 7,893,321
Lands WEM (1992) Biochemistry and physiology of n-3 fatty acids. J Fed Am Soc Exp Biol 6:2530–2536
Lands WEM (2005) Dietary fat and health: the evidence and the politics of prevention. Careful use of dietary fats can improve life and prevent disease. Ann NY Acad Sci 1055:179–192
Lee M, Lenman M, Banaś A, Bafor M, Singh S, Schweizer M, Nilsson R, Liljenberg C, Dahlqvist A, Gummeson P-O, Sjödahl S, Green A, Stymne S (1998) Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation. Science 280:915–918
Li R, Yu K, Hildebrand DF (2010) DGAT1, DGAT2 and PDAT expression in seeds and other tissues of epoxy and hydroxyl fatty acid accumulating plants. Lipids 45:147–157
Liu J-W, DeMichele S, Bergana M, Bobik E Jr, Hastilow C, Chuang L-T, Mukerji P, Huang Y-S (2001) Characterization of oil exhibiting high γ-linolenic acid from a genetically transformed canola strain. J Am Oil Chem Soc 78:489–493
Lu C, Xin Z, Ren Z, Miquel M, Browse J (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA 106:18837–18842
Menendez JA, Vellon L, Colomer R, Lupu R (2005) Effect of gammalinolenic acid on the transcriptional activity of the Her-2/neu (erB-2) ongogene. J Natl Cancer Inst 97(21):1611–1615
Metz JG, Kuner JM, Lippmeier JC, Moloney MM, Nykiforuk CL (2007) Polyunsaturated fatty acid production in heterologous organisms using PUFA polyketide synthase systems. WO-2007/106905
Moloney MM, Boothe J, Keon R, Nykiforuk C, and Van Rooijen G (2009) Methods for the production of insulin in plants. US Patent 7,547,821
Moloney MM, Reid A, Nykiforuk CL, Boothe JG (2010) Methods for the production of apolipoproteins in transgenic plants. US Patent 7,786,352
Mozaffarian D, Ascherio A, Hu FB, Stamfper MJ, Willet WC, Siscovick DS, Rimm EB (2005) Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 111:157–164
Mukerji P, Huang Y-S, Das T, Thurmond J, Pereira SL (2003) Desaturase genes and uses thereof. US Patent 6,635,451
Napier JA (2007) The production of unusual fatty acids in transgenic plants. Annu Rev Plant Biol 58:295–319
Napier JA, Graham IA (2010) Tailoring plant lipid composition: designer oilseeds come of age. Curr Opin Plant Biol 13:330–337
Napier JA, Beaudoin F, Michaelson LV, Sayanova O (2004) The production of long chain polyunsaturated fatty acids in transgenic plants by reverse-engineering. Biochimie 86:785–793
Nykiforuk CL, Boothe J, Murray E, Keon R, Goren J, Markley N, Moloney M (2006) Transgenic expression and recovery of biologically active recombinant human DesB30-insulin from Arabidopsis thaliana seeds. Plant Biotechnol J 4:77–85
Nykiforuk CL, Shen Y, Murray EW, Boothe JG, Busseil D, Rheume E, Tardif JC, Reid A, Moloney MM (2010) Transgenic expression and recovery of biologically active recombinant Apolipoprotein AI Milano from safflower (Carthamus tinctorius) seeds. Plant Biotechnol J 9:250–263
Okuyama H (2001) High n-6 to n-3 ratio of dietary fatty acids rather than serum cholesterol as a major risk factor for coronary heart disease. Eur J Lipid Sci Technol 103:418–422
Palombo JD, DeMichele SJ, Boyce PJ, Lydon EE, Liu J-W, Huang Y-S, Forse RA, Mizgerd JP, Bistrian BR (1999) Short-term enteral feeding with diets containing eicosapentaenoic and γ-linolenic acids modulates alveolar macrophage fatty acid composition and eicosanoid synthesis without compromising bactericidal function in rats. Crit Care Med 27:1908–1915
Poisson J-P, Narce M, Huang Y-S, Mills D (1996) γ-linolenic acid biosynthesis and chain elongation in fasting and diabetes mellitus. In: Huang Y-S, Mills DE (eds) γ-Linolenic acid, metabolism and its roles in nutrition and medicine. AOCS Press, pp 252–272
Rahamatalla AB, Babiker EE, Krishna AG, El Tinay AH (2001) Changes in fatty acids composition during seed growth and physiochemical characteristics of oil extracted from four safflower cultivars. Plant Foods Hum Nutr 56:385–395
Reddy AS, Thomas TL (1996) Expression of a cyanobacterial Δ6-desaturase gene results in γ-linolenic acid production in transgenic plants. Nat Biotechnol 14:639–642
Ruiz-López N, Haslam RP, Venegas-Calerón M, Larson TR, Graham IA, Napier JA, Sayanova O (2009) The synthesis and accumulation of stearidonic acid in transgenic plants: a novel source of ‘heart-healthy’ omega-3 fatty acids. Plant Biotechnol J 7:704–716
Runzhi L, Keshun Y, Hatanaka R, Hildebrand DF (2010) Veronia DGATs increase accumulation of epoxy fatty acids in oil. Plant Biotechnol J 8:184–195
Sato S, Xing A, Ye X, Schweiger B, Kinney A, Graef G, Clemente T (2004) Production of γ-linolenic acid and stearidoinic acid in seeds of marker-free transgenic soybean. Crop Sci 44:646–652
Sayanova O, Smith MA, Lapinskas P, Stobart AK, Dobson G, Christie WW, Shewry PR, Napier JA (1997) Expression of borage desaturase cDNA containing an N-terminal cytochrom b5 domain results in the accumulation of high levels of Δ6-desaturated fatty acids in transgenic tobacco. Proc Natl Acad Science USA 94:4211–4216
Sayanova O, Davies GM, Smith MA, Griffiths G, Stobart AK, Shewry PR, Napier JA (1999) Accumulation of Δ6-unsaturated fatty acids in transgenic tobacco plants expressing a Δ6-desaturase from Borago officinalis. J Exp Bot 50:1647–1652
Simopoulos AP (1999) Essential fatty acids in health and chronic disease. Am J Clin Nutr 70:560S–569S
Ståhl U, Ek B, Stymne S (1998) Purification and characterization of a low-molecular-weight phospholipase A2 from developing seeds of elm. Plant Physiol 117:197–205
Stobart AK, Stymne S (1985) The interconversion of diacylglycerol and phosphatidylcholine during triacylglycerol production in microsomal preparations of developing cotyledons of safflower (Carthamus tinctorius L.). Biochem J 232:217–221
Stoger E, Ma JK-C, Fischer R, Christou P (2005) Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol 16:167–173
Stymne S, Stobart AK (1984) Evidence for the reversibility of the acyl-CoA: lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem J 223:305–314
Stymne S, Stobart AK (1986) Biosynthesis of γ-linolenic acid in cotyledons and microsomal preparation of the developing seeds of common borage (Borago officinalis). Biochem J 24:385–393
Thomaeus S, Carlsson AS, Stymne S (2001) Distribution of fatty acids in polar and neutral lipids during seed development in Arabidopsis thaliana genetically engineered to produce acetylenic, epoxy and hydroxyl fatty acids. Plant Sci 161:997–1003
Traitler H, Wille HJ, Studer A (1988) Fractionation of black currant seed oil. J Am Oil Chem Soc 65:755–760
Tsevegsüren N, Aitzetmüller K, Vosmann K (1997) Unusual fatty acids in Compositae: y-linolenic acid in Saussurea spp. seed oils. J High Resolut Chromatogr 20:315–320
Tsevegsüren N, Aitzetmüller K, Vosmann K (1999) Occurrence of gamma-linolenic acid in compositae: a study of Youngia tenuicaulis seed oil. Lipids 34:525–529
Tsevegsüren N, Fujimoto K, Christie WW, Endo Y (2003) Occurrence of a novel cis, cis, cis-octadeca-3,9,12-trienoic (Z,Z,Z-octadeca-3,9,12-trienoic) acid in Chrysanthemum (Tanacetum) zawadskii herb (Compositae) seed oil. Lipids 38:573–578
Ul’chenko NT, Gigienova EI, Umarov AU, Isamukhamedov ASh (1981) Hydroxy acids of the seed oils of five plants of the family Asteraceae. Chem Nat Compd 17:26–30
Ursin VM (2003) Modification of plant lipids for human health: development of functional land-based omega-3 fatty acids. J Nutr 133:4271–4274
van Rooijen GJH, Moloney MM (1995) Structural requirements of oleosin domains for subcellular targeting to the oil body. Plant Physiol 109:1353–1361
Velasco L, Fernández-Martínez JM (2001) Breeding for oil quality in safflower. The Vth International Safflower Conference, Montana
Venegas-Calerón M, Sayanova O, Napier JA (2010) An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog Lipid Res 49:108–119
Vogel G, Browse J (1996) Cholinephosphotransferase and diacylglycerol acyltransferase. Plant Physiol 110:923–931
Weselake RJ, Pomeroy MK, Furukawa TL, Golden JL, Little DB, Laroche A (1993) Developmental profile of diacylglycerol acyltransferase in maturing seeds of oilseed rape and safflower and microspore-derived cultures of oilseed rape. Plant Physiol 102:565–571
Willett WC (2007) The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J Cardiovasc Med 8:S42–S45
Wu G, Truska M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X (2005) Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat Biotechnol 23:1013–1017
Yurchenko OP, Field CJ, Nykiforuk CL, Moloney MM, Weselake RJ (2007) Genetic modification of oilseeds to produce bioactive lipids. In: Acharya SN, Thomas JE (eds) Advances in medicinal plant research. Research Signpost, Kerala, pp 357–400
Acknowledgments
This work was conducted under an existing agreement between SemBioSys Genetics Inc. and Arcadia Biosciences for commercial applications protected under US patent application 20070067870 filed by Arcadia Biosciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11248_2011_9543_MOESM1_ESM.pdf
Figure S1. Plant binary vectors and strategies employed to accumulate GLA in safflower. (a) The plant binary expression vectors designed to express Δ6-fatty acid desaturase from Saprolegnia diclina (pSBS 4119), Δ6-fatty acid desaturase from Mortierella alpina (pSBS4763) or the double expression cassette encoding for both Δ6-fatty acid desaturase and Δ12-fatty acid desaturase from M. alpina (pSBS4766) under the transcriptional control of Arabidopsis oleosin promoter (OleoP) and terminator (OleoT) to ensure tightly controlled expression during seed development. Other genetic elements of the TDNA including RB, right border; UbiP, ubiquitin promoter, PAT, phosphinothricin acetyltransferase gene; UbiT, ubiquitin terminator; LB, left border are described in Experimental procedures. (b) Schematic of recombinant Δ6-fatty acid desaturase (Δ6 FAD) and Δ12-fatty acid desaturase (Δ12 FAD) activities introduced into high oleic (S317) and high linoleic (Centennial) backgrounds by Agrobacterium-mediated transformation as described in Experimental procedures within the context of endogenous Δ9-fatty acid desaturase (Δ9 FAD) and Δ12-fatty acid desaturase (Δ12 FAD) activity present in safflower and further described below. Supplementary material 1 (PDF 115 kb)
11248_2011_9543_MOESM2_ESM.pdf
Figure S2. Representative GLC chromatograms following the separation of FAMES on the HP-Innowax column (as described in Experimental procedures). (a) The identification of FAMES was performed using NuChek 502 GLC standard in combination with a retention time established with (b) mGLA standard (C18:3Δ6,9,12 cis) also purchased from NuChek (Elysian, MN, USA). Representative chromatograms are also provided for transgenic seed in the high linoleic acid variety (c) expressing pSBS4119 (S. diclina Δ6-FAD) in comparison to a (d) pSBS4119 null segregant (NS) and (e) expressing pSBS4763 (M. alpina Δ6-FAD) in comparison to a (f) pSBS4763 null segregant (NS). (g) A representative chromatogram of wild type Centennial (WT CENT) is also provided for comparisons between non-transformed seed, null segregants and transgenic seed. Representative chromatograms presented for transgenic seed in the high oleic acid variety (h) expressing the double expression cassette pSBS4766 (M. alpina Δ12-FAD and Δ6-FAD) in comparison to (i) a pSBS4766 null segregant (NS) and (j) non-transformed wild type S317 seed are also provided. Supplementary material 2 (PDF 83 kb)
11248_2011_9543_MOESM3_ESM.pdf
Figure S3. FAMEs analysis of the different classes of lipids between seed accumulating high-levels of GLA from pSBS4119 (S. diclina Δ6-FAD) transformations in comparison to the non-transformed wild-type Centennial seed in the separated lipid classes consisting of phospholipids (PL) and triacylgylcerol (TAG) separated by TLC prior to transmethylation as described in experimental procedures. Each assessment is the mean plus or minus one standard deviation (n = 3) and the *horizontal line with hanging arrows represents P values of less than 0.05 between LA (C18:2Δ9,12 cis) and GLA (C18:3Δ6,9,12 cis) for the PL analysis and OA (C18:1Δ9 cis), LA (C18:2Δ9,12 cis) and GLA (C18:3Δ6,9,12 cis) for the TAG analysis. Supplementary material 3 (PDF 15 kb)
Rights and permissions
About this article
Cite this article
Nykiforuk, C.L., Shewmaker, C., Harry, I. et al. High level accumulation of gamma linolenic acid (C18:3Δ6.9,12 cis) in transgenic safflower (Carthamus tinctorius) seeds. Transgenic Res 21, 367–381 (2012). https://doi.org/10.1007/s11248-011-9543-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-011-9543-5