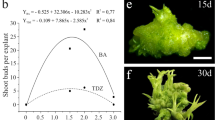
Abstract
Triploid nature of endosperm is the characteristic feature of angiosperms and is formed as a result of triple fusion. Present review discusses the morphogenic response and production of triploid plantlets by endosperm culture. Both mature and immature endosperm used for culture initiation responded differently in cultures. A key factor for the induction of cell divisions in mature endosperm cultures is the initial association of embryo but immature endosperms proliferate independent of embryo. In almost all the parasitic angiosperms, endosperm shows a tendency of direct differentiation of organs without prior callusing, whereas in autotrophic taxa the endosperm usually forms callus tissue followed by differentiation of shoot buds, roots or embryos. The endosperm tissue often shows a high degree of chromosomal variations and polyploidy. Mitotic irregularities, chromosome bridges and laggards are the other important characteristics of endosperm tissues. Triploids are usually seed sterile and is undesirable for plants where seeds are commercially useful. However, in cases where seedlessness is employed to improve the quality of fruits as in banana, apple, citrus, grapes, papaya etc. the induction of triploid plants would be of immense use. Triploid plants have more vigorous vegetative growth than their diploid counterparts. Hence, in plants where the vegetative parts are economically useful, triploids are of good use. This review focuses on the progress achieved so far in endosperm culture to obtain triploid plants.




Similar content being viewed by others
Abbreviations
- BA:
-
N6-Benzyladenine
- CH:
-
Casein hydrolysate
- CM:
-
Coconut milk
- CE:
-
Corn extract
- CWM:
-
Cow’s milk
- DAP:
-
Days after pollination
- 2, 4-D:
-
2, 4-Dichlorophenoxyacetic acid
- GA3 :
-
Gibberellic acid
- GJ:
-
Grape juice
- IBA:
-
Indole-3-butyric acid
- IAA:
-
Indole-3-acetic acid
- Kn:
-
6-furfurylamino purine (kinetin)
- MS:
-
Murashige and Skoog medium
- MT:
-
Murashige and Tucker medium
- NAA:
-
α-Naphthalene acetic acid
- PE:
-
Potato extract
- TDZ:
-
Thidiazuron
- TJ:
-
Tomato juice
- YE:
-
Yeast extract
- WM:
-
White’s medium
References
Bajaj YPS, Saini SS, Bidani M (1980) Production of triploid plants from the immature and mature endosperm cultures of rice. Theor Appl Genet 80:785–790
Bapat VA, Narayanaswamy S (1977) Mesocarp and endosperm culture of Achras sapota Linn. in vitro. Indian J Exp Biol 15:294–296
Battaglia E (1963) Apomixis. In: Maheshwari P (ed) Recent advances in the embryology of angiosperms. International Society for Plant Morphologists, University of Delhi, Delhi, pp 221–264
Bhojwani SS (1968) Mophogenetic studies on cultured mature endosperm and embryo of some angiosperms. Ph.D. thesis, University of Delhi, Delhi, 85pp
Bhojwani SS, Johri BM (1970) Cytokinin-induced shoot bud differentiation in mature endosperm of Scurrula pulverulenta. Z Pflanzenphysiol 63:269–275
Bhojwani SS, Johri BM (1971) Morphogenetic studies on cultured mature endosperm of Croton bonplandianum. New Phytol 70:761–766
Bhojwani SS, Razdan MK (1996) Plant tissue culture: theory and practice, a revised edition. Elsevier, Amsterdam, pp 1–766
Bottomley W, Kefford NP, Zwar JA, Goldacre PL (1963) Kinin activity from plant extracts. Aust J Biol Sci 16:395–406
Brown DJ, Canvin DT, Zilkey BF (1970) Growth and metabolism of Ricinus communis endosperm in tissue culture. Can J Bot 48:2323–2331
Ceniza MS, Ueda S, Sugimura Y (1992) In vitro culture of coconut endosperm: callus induction and its fatty acids. Plant Cell Rep 11:546–549
Chakraborty SP, Vijayan K, Roy BN, Qadri SMH (1998) In vitro induction of tetraploidy in mulberry (Morus alba L.). Plant Cell Rep 17:799–803
Chaturvedi R, Razdan MK, Bhojwani SS (2003) An efficient protocol for the production of triploid plants from endosperm callus of neem, Azadirchta indica A. Juss. J Plant Physiol 160:557–564
Cheema GS, Mehra PN (1982) Morphogenesis in endosperm cultures. In: Fujiwara A (ed) 5th international congress on plant tissue culture, Tokyo. The Japanese Association of Plant Tissue Culture, Tokyo, 230pp
Chikkannaiah PS, Gayatri MC (1974) Organogenesis in endosperm tissue cultures of Coedium varigatum Blume. Curr Sci 43:23–24
Dandin SB (1990) Biotechnology and host plant improvement in sericulture. Paper presented at the workshop on ‘Biotechnology in Sericulture’ held at Dept. of Biotechnology, Govt of India, New Delhi, 14pp
Das BC, Prasad DN, Sikdar AK (1970) Colchicine induced tetraploids of mulberry. Caryologia 23:283–293
Dwivedi NK, Sikdar AK, Dandin SB, Sastri CR, Jolly MS (1986) Induced tetraploids in mulberry. Morphological anatomical and cytological investigations in cv. RFS 135. Cytologia 51:393–401
Faranda S, Genga A, Viotti A, Manzocchi LA (1994) Stable transformed cell lines from protoplasts of maize endosperm suspension cultures. Plant Cell Tissue Organ Cult 37:39–46
Felker FC, Goodwin JC (1988) Sugar uptake by maize endosperm suspension cultures. Plant Physiol 88:1235–1239
Gamborg OL, Mitter RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Garg L, Bhandari NN, Rani V, Bhojwani SS (1996) Somatic embryos and regeneration of triploid plants in endosperm cultures of Acacia nilotica. Plant Cell Rep 15:855–858
Gayatri MC (1978) In vitro studies on Codiaeum variegatum: growth and organogenesis in endosperm tissue. Phytomorphology 28:395–400
Gmitter FG, Ling XB, Deng XX (1990) Induction of triploid plants from endosperm calli in vitro. Theor Appl Genet 80:785–790
Goralski G, Popielarska M, Slesak H, Siwinska D, Batycka M (2005) Organogenesis in endosperm of Actinidia deliciosa cv. Hayward cultured in vitro. Acta Biol Crac Ser Bot 47:121–128
Gui YL, Mu SK, Xu TY (1982) Studies on morphological differentiation of endosperm plants of Chinese gooseberry in vitro. Acta Bot Sin 24:216–221
Gu SR, Gui YI, Xu TY (1985) Induction of endosperm plantlets in Lycium. Acta Bot Sin 27:106–109
Hamada S (1963) Polyploid mulberry tree in practice. Indian J Ser 1:3–4
Ingale J, Hageman RH (1965) Metabolic changes associated with the germination of corn III. Effects of gibberellic acid on endosperm metabolism. Plant Physiol 40:672–675
Johnston SA, Den Nijs TPM, Peloquin SJ, Hanneman RE Jr (1980) The significance of genic balance to endosperm development in interspecific crosses. Theor Appl Genet 57:5–9
Johri BM, Bhojwani SS (1965) Growth response of mature endosperm in cultures. Nature 298:1345–1347
Johri BM, Bhojwani SS (1971) In vitro studies on morphogenesis of embryo and endosperm in parasitic angiosperms. J Indian Bot Soc 50:275–285
Johri BM, Nag KK (1970) Endosperm of Taxillus vestitus wall. A system to study the effect of cytokinins in vitro in shoot bud formation. Curr Sci 39:177–179
Johri BM, Nag KK (1974) Cytology and morphogenesis of embryo and endosperm tissues in Dendrophthoe and Taxillus. Cytologia 39:801–813
Johri BM, Srivastava PS (1972) In vitro growth response of mature endosperm of Ricinus communis L. In: Murty YS, Johri BM, Mohan Ram HY, Varghese TM (eds) Advances in plant morphology (Prof. Puri V Comm. Vol.). Sarita Prakashan, Meerut (India), pp 339–358
Johri BM, Srivastava PS (1973) Morphogenesis in endosperm cultures. Z Pflanzenphysiol 70:285–304
Kagan-Zur V, Mills D, Mizrahi Y (1990) Callus formation from tomato endosperm. Acta Hortic 280:139–142
Keller H, Wanner H, Baumann TW (1972) Caffeine synthesis in fruit and tissue cultures of Coffea arabica. Planta 108:339–350
Kin SM, Fraser LG, Harvey CF (1990) Initiation of plantlets from endosperm of Actinidia interspecific hybrids. Sci Hortic 44:107–117
Kumar PP, Raju CR, Chandra Mohan M, Iyer RD (1985) Induction and maintenance of friable callus from cellular endosperm of Cocos nucifera L. Plant Sci 40:203–207
Lakshmi Sita G, Raghva Ram NV, Vaidyanathan CS (1980) Triploid plants from endosperm culture of sandalwood by experimental embryogenesis. Plant Sci Lett 20:63–69
Lampe L, Mills CO (1933) Growth and development of isolated endosperm and embryo of maize. Abs Papers Bot Soc, Boston
Lampton RK (1952) Developmental and experimental morphology of the ovule and seed of Asimina triloba. Ph.D. thesis, Univ. Michigan, Ann Arbor, MI, 104pp
La Rue CD (1936) The growth of plant embryos in culture. Bull Torrey Bot Club 63:365–382
La Rue CD (1944) Regeneration of endosperm of gymnosperms and angiosperms. Am J Bot 36:798
La Rue CD (1947) Growth and regeneration of the endosperm of maize in culture. Am J Bot 34:585–586
La Rue CD (1949) Culture of the endosperm of maize. Am J Bot 36:798
Liu SQ, Liu JQ (1980) Callus induction and embryoid formation in endosperm culture of Prunus persica. Acta Bot Sin 22:198–199
Liu SQ, Gui YI, Gu SR, Xu TY (1987) Induction of endosperm callus and regeneration of endosperm plantlets of Asparagus officinalis. Acta Bot Sin 29:373–376
Machno D, Przywara L (1997) Endosperm culture of Actinidia spp. Acta Biol Crac Ser Bot 39:55–61
Manzocchi LA (1991) Stable endosperm cell suspension cultures from wild-type and opaque-2 maize. Plant Cell Rep 9:555–558
Masuda K, Koda Y, Okazawa Y (1977) Callus formation and embryogenesis of endosperm tissues of parsley seed cultured on hormone free medium. Physiol Plant 41:135–138
Mohan Ram HY, Satsangi A (1963) Induction of cell division in the mature endosperm of Ricinus communis during germination. Curr Sci 32:28–30
Monoco LC, Sondahi MR, Car Valho A, Crocomo OJ, Sharp WR (1977) Application of tissue culture in the improvement of coffee. In: Reinert J, Bajaj YPS (eds) Applied and fundamental aspects of plant cell tissue and organ culture. Springer Verlag, Berlin, pp 109–126
Mu SK, Liu SC (1978) Cytological observations on callus derived from apple endosperm cultured in vitro. In: Proceedings of symposium on plant tissue culture. Science Press, Peking, pp 507–510
Mu S, Liu S, Zhou Y, Quan N, Zhang P, Xie H, Zhang F, Yen Z (1977) Induction of callus from apple endosperm and differentiation of the endosperm plantlet. Sci Sin 55:370–376
Muniyamma M (1977) Triploid embryos from endosperm in vivo. Ann Bot 41:1077–1079
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murashige T, Tucker DPH (1969) Growth factor requirements of citrus tissue. In: Proceedings of the first international citrus symposium, pp 1155–1161
Nag KK, Johri BM (1971) Morphogenic studies on endosperm of some parasitic angiosperm. Phytomorphology 21:202–218
Nair S, Shirgurker MV, Mascarenhas AF (1986) Studies on endosperm culture of Annona squamosa Linn. Plant Cell Rep 5:132–135
Nakajima K (1962) Induction of useful mutations in mulberry by gamma radiation. JARQ 6:195–198
Nakano H, Tashiro T, Maeda E (1975) Plant differentiation in callus tissue induced from immature endosperm of Oryza sativa L. Z Pflanzenphysiol 76:444–449
Nitsch JP (1969) Experimental androgenesis in Nicotiana. Phytomorphology 19:389–404
Norstog K (1956) Growth of rye grass endosperm in vitro. Bot Gaz 117:253–259
Norstog K, Wall WE, Howland GP (1969) Cytological characteristics of ten-year-old rye grass endosperm tissue cultures. Bot Gaz 130:83–86
Ogawa Y (1964) Changes in the amount of gibberellin like substances in the seedling of Pharbitis nil with special reference to expansion of cotyledon. Plant Cell Physiol Tokyo 5:11–20
Raghuramalu Y (1989) Anther and endosperm culture of coffee. J Coffee Res 19:71–81
Rangaswamy NS, Rao PS (1963) Experimental studies on Santalum album L. Establishment of tissue culture of endosperm. Phytomorphology 13:450–454
Sehgal CB (1969) Experimental studies on maize endosperm. Beitr Biol Pflanz 46:233–238
Sehgal CB (1974) Growth of barley and wheat endosperm in cultures. Curr Sci 43:38–40
Sehgal CB, Abbas NS (1996) Induction of triploid plantlets from the endosperm culture of Mallotus philippensis mull. Arg Phytomorphol 46:283–289
Sehgal CB, Khurana S (1985) Morphogenesis and plant regeneration from cultured endosperm of Emblica officinalis Gaertn. Plant Cell Rep 4:263–266
Sehgal CB, Narang KH, Sunila A (1981) Growth responses of mature endosperm of Euphorbia geniculata Orteg. in cultures. Beitr Biol Pflanz 55:385–392
Sethi M, Rangaswami NS (1976) Endosperm embryoids in cultures of Nigella damascena. Curr Sci 45:109–111
Shihkin M, Shuchiung L (1977) Cytological observation on callus derived from apple endosperm cultured in vitro. In: Proceedings of symposium on plant tissue culture, May 25–30, Peking, pp 126–127
Sikdar AK, Jolly MS (1994) Induced polyploid in mulberry (Morus alba L.) I. Induction of tetraploids. Sericologia 34:105–116
Sikdar AK, Jolly MS (1995) Induced polyploid in mulberry (Morus spp.) II. Production of triploids and their yield evaluation. Bull Sericult Res 6:39–46
Smith MM, Stone BA (1973) Studies on Lolium multiflorum endosperm in tissue culture. Aust J Bio Sci 26:123–133
Srivastava PS (1971a). In vitro induction of triploid roots and shoots from mature endosperm of Jatropa panduraefolia. Z Pflanzenphysiol 66:93–96
Srivastava PS (1971b) In vitro growth requirements of mature endosperm of Ricinus communis. Curr Sci 40:337–339
Srivastava PS (1973) Formartion of triploid plantlets in endosperm cultures of Putranjiva roxburghii Z Pflanzenphysiol 69:270–273
Sternheimer EP (1954) Method of culture and growth of maize endosperm in vitro. Bull Torrey Bot Club 81:111–113
Straus J (1954) Maize endosperm tissue grown in vitro II. Morphology and cytology. Am J Bot 41:833–839
Straus J (1960) Maize endosperm tissue grown in vitro. III. Development of a synthetic medium. Am J Bot 47:641–647
Straus J, La Rue CD (1954) Maize endosperm tissue grown in vitro 1. Culture requirements. Am J Bot 41:687–694
Sun CS, Chu CC (1981) The induction of endosperm plantlets and their ploidy of barley in vitro. Acta Bot Sin 23:265
Syed Abbas N (1993) Studies on somatic embryogenesis and organogenesis in three economic plants, Mallotus philippensis, Aleurites fordii-mature endosperm, Trachyspermum ammi, seedling explants. Thesis submitted to University of Delhi, Delhi, India
Tamaoki T, Ullstrup JA (1958) Cultivation in vitro of excised endosperm and meristem tissues of corn. Bull Torrey Bot Club 85:260–272
Thomas TD, Bhatnagar AK, Bhojwani SS (2000) Production of triploid plants of mulberry (Morus alba L.) by endosperm culture. Plant Cell Rep 19:395–399
Tulecke W, Mc Granaham G, Ahmadi H (1988) Regeneration by somatic embryogenesis of triploid plants from endosperm of walnut, Juglans regia L. Cv. Manregian. Plant Cell Rep 7:301–304
Verma RC, Sarkar A, Sarkar S (1986) Induced amphidiploids in mulberry. Curr Sci 55:1203–1204
Wang TY, Chang CJ (1978) Triploid citrus plantlet from endosperm culture. Proceedings of symposium on plant tissue culture, pp 463–468
White PR (1963) The cultivation of animal and plant cells, 2nd edn. The Ronald Press, New York, 228pp
Williams EG, De Latour G (1980) The use of embryo culture with transplanted nurse endosperm for the production of interspecific hybrids in pasture legumes. Bot Gaz 141:252–257
Zhao H (1988) Induction of endosperm plantlets of ‘Jinfeng’ pear in vitro and their ploidy. In: Genetic manipulation of crops. Cassell Tycooly (UK), pp 370–371
Zhu Q, Chen X, Li W, Chen Y (1988) In vitro regeneration of plantlets from immature endosperm of maize (Zea mays). In: Genetic manipulation of crops. Cassell Tycooly (UK), pp 370–371
Zur VK, Mills D, Mizrahi Y (1990) Callus formation from tomato endosperm. Acta Hortic 280:139–141
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, T.D., Chaturvedi, R. Endosperm culture: a novel method for triploid plant production. Plant Cell Tiss Organ Cult 93, 1–14 (2008). https://doi.org/10.1007/s11240-008-9336-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9336-6