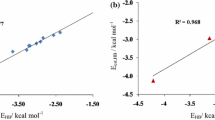
Abstract
Investigation of characteristics of hydrogen bonding between pyridine and water by MP2/aug-cc-pvdz method reveals that these two molecules may form three types of hydrogen bonds depending on nature of proton withdrawal site of pyridine. Change of orientation of water with respect to plane of aromatic ring leads to transformation of the O–H···N bond to O–H···π bond via wide region of the potential energy surface where both lone pair of the nitrogen atom and π-system make significant contribution into hydrogen bonding. Hydrogen bond in this intermediate region may be considered as mixed O–H···N/O–H···π bond representing new type of H bonds.



Similar content being viewed by others
References
Scheiner S (1997) Hydrogen bonding. A theoretical perspective. Oxford University Press, New York
Grabowski S (ed) (2006) Hydrogen bonding: new insights. Challenges & advances in computational chemistry & physics, vol 3, Springer, Dordrecht
Meot-Ner (Mautner) M (2005) Chem Rev 105:213. doi:10.1021/cr9411785
Steiner T (2002) Angew Chem Int Ed 41:48. doi:10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, Oxford
Sukhanov OS, Shishkin OV, Gorb L, Podolyan Y, Leszczynski J (2003) J Phys Chem B 107:2846. doi:10.1021/jp026487a
Sukhanov OS, Shishkin OV, Gorb L, Leszczynski J (2008) Struct Chem 19:171. doi:10.1007/s11224-007-9266-7
Boys SF, Bernardi F (1970) Mol Phys 19:553. doi:10.1080/00268977000101561
Meumier A, Levy B, Berthier G (1973) Theor Chim Acta 29:49. doi:10.1007/BF00528166
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.01, Gaussian Inc., Wallingford, CT
Weinhold F (1998) Natural bond orbital methods. In: Encyclopedia of computational chemistry, vol 3. John Wiley & Sons, Chichester, p 1792
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899. doi:10.1021/cr00088a005
King BF, Weinhold F (1995) J Chem Phys 103:333. doi:10.1063/1.469645
Weinhold F (1997) J Mol Struct THEOCHEM 398:181. doi:10.1016/S0166-1280(96)04936-6
Wong NB, Cheung YS, Wu DY, Ren Y, Tian AM, Li WK (2000) J Mol Struct THEOCHEM 507:153. doi:10.1016/S0166-1280(99)00386-3
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2005) NBO 5.0. Theoretical Chemistry Institute. University of Wisconsin, Madison, USA
Bader RWF (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Biegler-König F, Schönbohm J, Bayles D (2001) J Comput Chem 22:545. doi:10.1002/1096-987X(20010415)22:5<545::AID-JCC1027>3.0.CO;2-Y
Espinosa E, Molins E, Lecomte C (1998) Chem Phys Lett 285:170. doi:10.1016/S0009-2614(98)00036-0
Lyssenko KA, Korlyukov AA, Golovanov DG, Ketkov SY, Antipin MY (2006) J Phys Chem A 110:6543. doi:10.1021/jp057516v
Lyssenko KA, Antipin MY (2006) Russ Chem Bull 55:1. doi:10.1007/s11172-006-0208-0
Destexhe A, Smets J, Adamowicz L, Maes G (1994) J Phys Chem 98:1506. doi:10.1021/j100056a023
Dkhissi A, Adamowicz L, Maes G (2000) J Phys Chem A 104:2112. doi:10.1021/jp9938056
Smets J, McCarthy W, Maes G, Adamowicz L (1999) J Mol Struct 476:27. doi:10.1016/S0022-2860(98)00536-5
Cambridge Crystal Structure Database. Release (2008)
Baxter PNW, Connor JA, Wallis JD, Povey DC, Powell AK (1992) J Chem Soc Perkin Trans 1 1601. doi:10.1039/p19920001601
Langer P, Hoffmann HMR (1997) Tetrahedron 53:9145. doi:10.1016/S0040-4020(97)00609-1
Langer P, Hoffmann HMR, Wartchow R (1998) Z Kristallogr New Cryst Struct 213:193
Opozda EM, Lasocha W, Wlodarczyk-Gajda B (2006) J Mol Struct 784:149. doi:10.1016/j.molstruc.2005.08.034
Larson SB, Sanghvi YS, Revankar GR, Robins RK (1990) Acta Crystallogr C 46:791. doi:10.1107/S0108270189008498
Padilla-Martinez II, Martinez-Martinez FJ, Garcia-Baez EV, Torres-Valencia JM, Rojas-Lima S, Hopfl H (2001) J Chem Soc Perkin Trans 2 181
Acknowledgments
This work was supported by the NSF CREST Grant No. HRD–0318519.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shishkin, O.V., Konovalova, I.S., Gorb, L. et al. Novel type of mixed O–H···N/O–H···π hydrogen bonds: monohydrate of pyridine. Struct Chem 20, 37–41 (2009). https://doi.org/10.1007/s11224-009-9412-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9412-5