Abstract
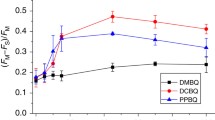
Arginine257 (R257), in the de-helix that caps the QB site of the D1 protein, has been shown by mutational studies to play a key role in the sensitivity of Photosystem II (PS II) to bicarbonate-reversible binding of the formate anion. In this article, the role of this residue has been further investigated through D1 mutations (R257E, R257Q, and R257K) in Chlamydomonas reinhardtii. We have investigated the activity of the QB site by studying differences from wild type on the steady-state turnover of PS II, as assayed through chlorophyll (Chl) a fluorescence yield decay after flash excitation. The effects of p-benzoquinone (BQ, which oxidizes reduced QB, Q −B ) and 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU, which blocks electron flow from Q −A to QB) were measured. The equilibrium constants of the two-electron gate were obtained through thermoluminescence measurements. The thermoluminescence properties were changed in the mutants, especially when observed after pretreatment with 100 μM BQ. A theoretical analysis of the thermoluminescence data, based mainly on the recombination pathways model of Rappaport et al. (2005), led to the conclusion that the free-energy difference for the recombination of Q −B with S2 was reduced by 20–40 mV in the three mutants (D1-R257K, D1-R257Q, and D1-R257E); this was interpreted to be due to a lowering of the redox potential of QB/Q −B . Further, since the recombination of Q −A with S2 was unaffected, we suggest that no significant change in redox potential of QA/Q −A occurred in these three mutants. The maximum variable Chl a fluorescence yield is lowered in the mutants, in the order R257K > R257Q > R257E, compared to wild type. Our analysis of the binary oscillations in Chl a fluorescence following pretreatment of cells with BQ showed that turnover of the QB site was relatively unaffected in the three mutants. The mutant D1-R257E had the lowest growth rate and steady-state activity and showed the weakest binary oscillations. We conclude that the size and the charge of the amino acid at the position D1-257 play a role in PS II function by modulating the effective redox potential of the QB/Q −B pair. We discuss an indirect mechanism mediated through electrostatic and/or surface charge effects and the possibility of more pleiotropic effects arising from decreased stability of the D1/D2 and D1/CP47 interfaces.









Similar content being viewed by others
Abbreviations
- BQ:
-
p-Benzoquinone
- C. reinhardtii :
-
Chlamydomonas reinhardtii
- Chl:
-
Chlorophyll
- 18-Crown-6:
-
Dicyclohexano-hexaoxacyclooctadecane
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- DMBQ:
-
2,5-Dimethyl-p-benzoquinone
- HS:
-
High salt culture medium
- ms:
-
Millisecond(s)
- PS II:
-
Photosystem II
- Ph:
-
Pheophytin
- QA :
-
Primary plastoquinone electron acceptor of Photosystem II
- QB :
-
Secondary plastoquinone electron acceptor of Photosystem II
- TAP:
-
Tris–acetate–phosphate culture medium
References
Amesz J, Fork DC (1967) Quenching of chlorophyll fluorescence by quinones in algae and chloroplasts. Biochim Biophys Acta 143:97–107. doi:10.1016/0005-2728(67)90114-4
Blubaugh DJ, Govindjee (1988) The molecular mechanism of the bicarbonate effect at the plastoquinone reductase site of photosynthesis. Photosynth Res 19:85–128. doi:10.1007/BF00114571
Bowes JM, Crofts AR (1980) Binary oscillations in the rate of reoxidation of the primary acceptor of Photosystem-II. Biochim Biophys Acta 590:373–384. doi:10.1016/0005-2728(80)90208-X
Bowes JM, Crofts AR (1981) Effect of DBMIB on the secondary electron acceptor B of Photosystem II. Arch Biochem Biophys 209:682–686. doi:10.1016/0003-9861(81)90329-5
Bowyer J, Hilton M, Whitelegge J, Jewess P, Camilleri P, Crofts A et al (1990) Molecular modeling studies on the binding of phenylurea inhibitors to the D1 protein of Photosystem II. Z Naturforsch 45c:379–387
Bouges-Bocquet B (1973) Electron transfer between two photosystems in spinach chloroplasts. Biochim Biophys Acta 314:250–256. doi:10.1016/0005-2728(73)90140-0
Crofts AR, Wraight CA, Fleischman DE (1971) Energy conservation in the photochemical reactions of photosynthesis and its relation to delayed fluorescence. FEBS Lett 15:89–100. doi:10.1016/0014-5793(71)80031-5
Crofts AR, Robinson HH, Andrews K, Van Doren S, Berry E (1987) Catalytic sites for reduction and oxidation of quinones. In: Papa S, Chance B, Ernster L (eds) Cytochrome systems: molecular biology and bioenergetics. Plenum Publ, New York, pp 617–624
Crofts AR, Baroli I, Kramer D, Taoka S (1993) Kinetics of electron transfer between QA and QB in wild-type and herbicide-resistant mutants of C. reinhardtii. Z Naturforsch 48c:259–266
Cuni A, Xiong L, Sayre R, Rappaport F, Lavergne J (2004) Modification of the pheophytin midpoint potential in Photosystem II: modulation of the quantum yield of charge separation and of charge recombination pathways. Phys Chem Chem Phys 6:4825–4831. doi:10.1039/b407511k
De Vault D, Govindjee (1990) Photosynthetic glow peaks and their relationship with the free-energy changes. Photosynth Res 24:175–181
De Vault D, Govindjee, Arnold W (1983) Energetics of photosynthetic glow peaks. Proc Natl Acad Sci USA 80:983–987. doi:10.1073/pnas.80.4.983
Ducruet J-M, Peeva V, Havaux M (2007) Chlorophyll thermofluorescence and thermoluminescence as complementary tools for the study of stress in plants. Photosynth Res 93:159–171. doi:10.1007/s11120-007-9132-x
Eaton-Rye JJ, Govindjee (1988a) Electron transfer through the quinone acceptor complex of Photosystem II in bicarbonate-depleted spinach thylakoid membranes as a function of actinic flash number and frequency. Biochim Biophys Acta 935:237–247. doi:10.1016/0005-2728(88)90220-4
Eaton-Rye JJ, Govindjee (1988b) Electron transfer through the quinone acceptor complex of Photosystem II after one or two actinic flashes in bicarbonate-depleted spinach thylakoid membranes. Biochim Biophys Acta 935:248–257. doi:10.1016/0005-2728(88)90221-6
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303:1831–1838. doi:10.1126/science.1093087
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 54:1665–1669. doi:10.1073/pnas.54.6.1665
Govindjee, Van Rensen JJS (1993) Photosystem II reaction centers and bicarbonate. In: Deisenhofer J, Norris JR (eds) Photosynthetic reaction centers, vol 1. Academic, Orlando, pp 357–389
Govindjee, Pulles MPJ, Govindjee R, van Gorkom HJ, Duysens LNM (1976) Inhibition of the reoxidation of the secondary electron acceptor of Photosystem II by bicarbonate depletion. Biochim Biophys Acta 449:602–605
Harris EH (1988) The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use. Academic, San Diego
Humphrey W, Dalke A, Schulten K (1996) VMD—visual molecular dynamics. J Mol Graph 14:33–38. doi:10.1016/0263-7855(96)00018-5
Inoue Y (1996) Photosynthetic thermoluminescence as a simple probe of Photosystem II electron transport. In: Amesz J, Hoff AJ (eds) Biophysical techniques in photosynthesis, advances in photosynthesis and respiration, vol 3 (series editor: Govindjee). Kluwer Academic Publishers (now Springer), Dordrecht, pp 93–107
Kern J, Renger G (2007) Photosystem II: structure and mechanism of water:plastoquinone oxidoreductase. Photosynth Res 94:183–202. doi:10.1007/s11120-007-9201-1
Keranen M, Pulo P, E-M Aro, Govindjee, Tyystjärvi E (1998) Thermoluminescence B and Q bands are the same temperature in an autotrophic and a heterotrophic D1 protein mutant of Synechocystis sp. PCC 6803. In: Garab G (ed) Photosynthesis mechanisms and effects. Kluwer Academic Publishers (now Springer), Dordrecht, pp 1145–1148
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution—I. A linear four step mechanism. Photochem Photobiol 11:457–475. doi:10.1111/j.1751-1097.1970.tb06017.x
Kramer DM, Crofts AR (1990) A portable multi-flash fluorimeter for measurement of donor and acceptor reactions of Photosystem 2 in leaves of intact plants under field conditions. Photosynth Res 26:181–193. doi:10.1007/BF00033131
Kramer D, Adawi O, Morse PII, Crofts AR (1987) A portable double-flash spectrophotometer for measuring the kinetics of electron transport components in intact leaves. In: Biggins J (ed) Progress in photosynthesis research, vol 2. Martinus Nijhoff Publishers, Dordrecht, pp 665–668
Kramer DM, Roffey RA, Sayre RT, Govindjee (1994) The A(T) thermoluminescence band from Chlamydomonas reinhardtii and the effects of mutagenesis of histidine-residues on the donor side of the Photosystem II D1 polypeptide. Biochim Biophys Acta 1185:228–237. doi:10.1016/0005-2728(94)90214-3
Lavergne J (1982) Interaction of exogenous benzoquinone with Photosystem II in chloroplasts: the semiquinone form acts as a dichlorophenyldimethylurea-insensitive secondary acceptor. Biochim Biophys Acta 679:12–18. doi:10.1016/0005-2728(82)90249-3
Lavergne J (1984) Absorption changes of Photosystem II donors and acceptors in algal cells. FEBS Lett 173:9–14. doi:10.1016/0014-5793(84)81006-6
Lavergne J, Briantais JM (1996) Photosystem II heterogeneity. In: Ort DR, Yocum C (eds) Oxygenic photosynthesis: the light reactions. Advances in photosynthesis and respiration (series editor: Govindjee), vol 4. Kluwer Academic Publishers (now Springer), Dordrecht, pp 265–287
Lavergne J, Leci E (1993) Properties of inactive Photosystem II centers. Photosynth Res 35:323–343. doi:10.1007/BF00016563
Lavorel J (1968) Sur une relation entre fluorescence et luminescence dans les systemes photosynthetique. Biochim Biophys Acta 153:727–730. doi:10.1016/0005-2728(68)90203-X
Loll B, Kern J, Saenger W, Zouni A, Biesadka J (2005) Towards complete cofactor arrangement in the 3 0 Å resolution structure of Photosystem II. Nature 438:1040–1044. doi:10.1038/nature04224
Mäenpää P, Miranda T, Tyystjärvi E, Govindjee, Tyystjärvi T, Ducruet J-M, Etienne A-L, Kirilovsky D (1995) A mutation in the D-de loop of D1 modifies the stability of the S2Q −A and S2Q −B states in Photosystem II. Plant Physiol 107:187–197
Minagawa J, Crofts AR (1994) A robust protocol for site-directed mutagenesis of the D1 protein of Chlamydomonas reinhardtii: a PCR-spliced psbA gene in a plasmid conferring spectinomycin resistance was introduced into a psbA deletion strain. Photosynth Res 42:121–131. doi:10.1007/BF02187123
Moser CC, Page CC, Chen X, Dutton PL (1997) Biological electron tunneling through native protein media. J Biol Inorg Chem 2:393–398. doi:10.1007/s007750050149
Moser CC, Page CC, Dutton PL (2006) Darwin at the molecular scale: selection and variance in electron tunneling proteins including cytochrome c oxidase. Philos Trans R Soc 361:1295–1305. doi:10.1098/rstb.2006.1868
Mulo P, Tyystjärvi T, Tyystjärvi E, Govindjee, Mäenpää P, Aro E-M (1997) Mutagenesis of the D-E Loop of Photosystem II reaction centre protein D1: function and assembly of Photosystem II. Plant Mol Biol 33:1059–1071. doi:10.1023/A:1005765305956
Papageorgiou G, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration (series editor: Govindjee), vol 19. Springer, Dordrecht
Petrouleas V, Crofts AR (2005) The iron-quinone acceptor complex. In: Wydrzynski T, Satoh K (eds) Photosystem II: the light-driven water:plastoquinone oxidoreductase. Advances in photosynthesis and respiration (series editor: Govindjee), vol 22. Springer, Dordrecht, pp 177–206
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents—verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975:384–394. doi:10.1016/S0005-2728(89)80347-0
Randall JT, Wilkins MHF (1945) Phosphorescence and electron traps I. The study of trap distributions. Proc R Soc A 184:365–389. doi:10.1098/rspa.1945.0024
Rappaport F, Guergova-Kuras M, Nixon PJ, Diner BA, Lavergne J (2002) Kinetics and pathways of charge recombination in Photosystem II. Biochemistry 41:8518–8527. doi:10.1021/bi025725p
Rappaport F, Cuni A, Xiong L, Sayre R, Lavergne RM (2005) Charge recombination and thermoluminescence in Photosystem II. Biophys J 88:1948–1958. doi:10.1529/biophysj.104.050237
Robinson HH, Crofts AR (1983) Kinetics of the oxidation reduction reactions of the Photosystem II quinone acceptor complex, and the pathway for deactivation. FEBS Lett 153:221–226. doi:10.1016/0014-5793(83)80152-5
Ruffle SV, Donnelly D, Blundell TL, Nugent JHA (1992) A 3-dimensional model of the Photosystem II reaction center of Pisum sativum. Photosynth Res 34:287–300. doi:10.1007/BF00033446
Rutherford AW, Crofts AR, Inoue Y (1982) Thermoluminescence as a probe of Photosystem II photochemistry—the origin of the flash-induced glow peaks. Biochim Biophys Acta 682:457–465. doi:10.1016/0005-2728(82)90061-5
Rutherford W, Govindjee, Inoue Y (1984) Charge accumulation and photochemistry in leaves studied by thermoluminescence and delayed light emission. Proc Natl Acad Sci USA 81:1107–1111. doi:10.1073/pnas.81.4.1107
Sane PV, Rutherford AW (1986) Thermoluminescence from photosynthetic membranes. In: Govindjee, Amesz J, Fork DC (eds) Light emission by plants and bacteria. Academic, Orlando, pp 329–360
Sarkar G, Sommer SS (1990) The megaprimer method of site-directed mutagenesis. Biotechniques 8:404–407
Shinkarev VP, Wraight CA (1993) Oxygen evolution in photosynthesis—from unicycle to bicycle. Proc Natl Acad Sci USA 90:1834–1838. doi:10.1073/pnas.90.5.1834
Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 46:83–91. doi:10.1073/pnas.46.1.83
Taoka S (1989) Kinetics of electron transfer and binding of inhibitors in the two electron gate of chloroplasts. Ph.D. thesis, University of Illinois, Urbana-Champaign
Taoka S, Crofts AR (1987) Competition of Inhibitors with the secondary quinone in dark-adapted thylakoid membranes. In: Biggins J (ed) Progress in photosynthesis research, vol 2. Martinus Nijhoff Publishers, Dordrecht, pp 425–428
Taoka S, Robinson HH, Crofts AR (1983) Kinetics of the reactions of the two-electron gate of photosystem: studies of the competition between plastoquinone and inhibitors. In: Inoue Y, Crofts AR, Govindjee, Murata N, Renger G, Satoh K (eds) The oxygen evolving system of photosynthesis. Academic, Tokyo, pp 369–382
Van Rensen JJS (2005) Role of bicarbonate at the acceptor side of Photosystem II. In: Govindjee, Beatty JH, Gest H, Allen JF (eds) Discoveries in photosynthesis. Advances in photosynthesis and respiration (series editor: Govindjee), vol 20. Springer, Dordrecht, pp 303–310
Van Rensen JJS, Xu C, Govindjee (1999) Role of bicarbonate in the Photosystem II, the water-plastoquinone oxido-reductase of plant photosynthesis. Physiol Plant 105:585–592. doi:10.1034/j.1399-3054.1999.105326.x
Vass I (2003) The history of photosynthetic thermoluminescence. Photosynth Res 76:303–318. doi:10.1023/A:1024989519054
Vass I, Govindjee (1996) Thermoluminescence from the photosynthetic apparatus. Photosynth Res 48:117–126. doi:10.1007/BF00041002
Vassiliev S, Bruce D (2008) Toward understanding molecular mechanisms of light harvesting and charge separation in Photosystem II. Photosynth Res: 15 pp. doi:10.007/s11120-008-9203-4
Velthuys BR (1981) Electron-dependent competition between plastoquinone and inhibitors for binding to Photosystem II. FEBS Lett 126:277–281. doi:10.1016/0014-5793(81)80260-8
Velthuys BR, Amesz J (1974) Charges accumulation at the reducing side of system 2 of photosynthesis. Biochim Biophys Acta 333:85–94. doi:10.1016/0005-2728(74)90165-0
Vernotte C, Briantais J-M, Astier C, Govindjee (1995) Differential effects of formate in single and double mutants of D1 in Synechocystis species PCC 6714. Biochim Biophys Acta 1229:296–301. doi:10.1016/0005-2728(95)00018-E
Wollman F-A (1978) Determination and modification of the redox state of the secondary acceptor of Photosystem II in the dark. Biochim Biophys Acta 503:263–273. doi:10.1016/0005-2728(78)90187-1
Wraight CA (1981) Oxidation-reduction physical chemistry of the acceptor quinone complex in bacterial photosynthetic reaction centers: evidence for a new model of herbicide activity. Isr J Chem 21:348–354
Wydrzynski TJ, Satoh K (eds) (2005) Photosystem II: the light-driven water:plastoquinone oxidoreductase. Advances in photosynthesis and respiration (series editor: Govindjee), vol 22. Springer, Dordrecht
Xiong J, Subramaniam S, Govindjee (1996) Modeling of the D1/D2 proteins and cofactors of the Photosystem II reaction center: implications for herbicide and bicarbonate binding. Protein Sci 5:2054–2073
Xiong J, Minagawa J, Crofts A, Govindjee (1998a) Loss of inhibition by formate in newly constructed Photosystem II D1 mutants, D1-R257E and D1-R257M, of Chlamydomonas reinhardtii. Biochim Biophys Acta 1365:473–491. doi:10.1016/S0005-2728(98)00101-7
Xiong J, Subramaniam S, Govindjee (1998b) A knowledge-based three dimensional model of the Photosystem II reaction center of Chlamydomonas reinhardtii. Photosynth Res 56:229–254. doi:10.1023/A:1006061918025
Acknowledgments
Govindjee acknowledges support from the Department of Plant Biology, University of Illinois at Urbana-Champaign; ARC and SWR acknowledge support from NIH GM35438; JM acknowledges support from MEXT 18GS0318. MS acknowledges support from the Cooperative State Research, Education and Extension Service, U.S. Department of Agriculture, Project No. ILLU-875-389. We thank Jan Kern for discussions on the location of bicarbonate ions on the Photosystem II reaction center. We also thank George Papageorgiou for reading the final draft of this manuscript and for making valuable suggestions to improve the readability of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jun Minagawa and Manfredo Seufferheld had equal credit in this work.
Rights and permissions
About this article
Cite this article
Rose, S., Minagawa, J., Seufferheld, M. et al. D1-arginine257 mutants (R257E, K, and Q) of Chlamydomonas reinhardtii have a lowered QB redox potential: analysis of thermoluminescence and fluorescence measurements. Photosynth Res 98, 449–468 (2008). https://doi.org/10.1007/s11120-008-9351-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-008-9351-9