Abstract
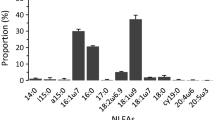
Nitrogen isotope values (δ15N) are higher in ectomycorrhizal fungi than in their plant hosts but the wide variability in δ15N among sporocarps of different fungal taxa is unexplained. We propose that fungal δ15N reflects sequestration of fungal nitrogen to build fungal biomass, and should accordingly reflect fungal exploration strategies and hyphal properties. To test this, we compared δ15N to exploration types, hyphal hydrophobicity, and the presence of rhizomorphs in ectomycorrhizal species from surveys at four sites in temperate and boreal coniferous forests. Fungi with exploration types of high biomass, such as long-distance (e.g., Suillus), medium-distance mat (e.g., Hydnellum), and medium-distance fringe (e.g., Cortinarius) were 4–7‰ more enriched in 15N than fungi with exploration types of low biomass [medium-distance smooth (e.g., Amanita), short-distance (e.g., Inocybe), and contact (e.g., Hygrophorus)]. High biomass types comprised 79% (Åheden, northern Sweden), 65% (Deer Park, Pacific Northwest, USA), 45% (Stadsskogen, central Sweden), and 39% (Hoh, Pacific Northwest, USA) of ectomycorrhizal species, with these types more prevalent at sites of lower nitrogen availability. Species with hydrophobic hyphae or with rhizomorphs were 3–4‰ more enriched in 15N than taxa with hydrophilic hyphae or without rhizomorphs. The consistency of these patterns suggest that δ15N measurements could provide insights into belowground functioning of poorly known taxa of ectomycorrhizal fungi and into relative fungal biomass across ectomycorrhizal communities.





Similar content being viewed by others
References
Agerer R (2001) Exploration types of ectomycorrhizae. A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107–114. doi:10.1007/s005720100108
Agerer R (2006) Fungal relationships and structural identity of their ectomycorrhizae. Mycol Prog 5:67–107. doi:10.1007/s11557-006-0505-x
Agerer R (2007) Diversity of ectomycorrhizae as seen from below and above ground: the exploration types. Z Mykol 73:61–88
Agerer R, Raidl S (2004) Distance related half-quantitative estimation of the extramatrical ectomycorrhizal mycelia of Cortinarius obtusus and Tylopora asterophora. Mycol Prog 3:57–64. doi:10.1007/s11557-006-0077-9
Ågren GI, Bosatta E (1996) Theoretical ecosystem ecology. Cambridge University Press, Cambridge
Arnolds E (1991) Decline of ectomycorrhizal fungi in Europe. Agric Ecosyst Environ 35:209–244. doi:10.1016/0167-8809(91)90052-Y
Bardin R, Domanach A-M, Chalamet A (1977) Rapports isotopiques naturels de l’azote. II. Variations d’abondance du 15 N dans les échantillons de sols et plants; applications. Rev Ecol Biol Sol 14:395–402
Becerra A, Daniele G, Dominguez L, Nouhra E, Horton T (2002) Ectomycorrhizae between Alnus acuminata H.B.K. and Naucoria escharoides (Fr.:Fr.) Kummer from Argentina. Mycorrhiza 12:61–66
Boström B, Comstedt D, Ekblad A (2007) Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 153:89–98. doi:10.1007/s00442-007-0700-8
Dickie IA, Moyersoen B (2008) Towards a global view of ectomycorrhizal ecology. New Phytol 180:263–265. doi:10.1111/j.1469-8137.2008.02635.x
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6:121–126. doi:10.1016/S1360-1385(01)01889-1
Garten CT, Van Miegroet H (1994) Relationships between soil nitrogen dynamics and natural 15 N abundance in plant foliage from Great Smoky Mountains National Park. Can J Res 24:1636–1645. doi:10.1139/x94-212
Gebauer G, Taylor AFS (1999) 15 N natural abundance in fruit bodies of different functional groups of fungi in relation to substrate utilization. New Phytol 142:93–101. doi:10.1046/j.1469-8137.1999.00373.x
Hobbie EA (2005) Using isotopic tracers to follow carbon and nitrogen cycling of fungi. In: Dighton J, Oudemans P, White J (eds) The fungal community: its organization and role in the ecosystem. Marcel Dekker, New York, pp 361–381
Hobbie EA (2006) Carbon allocation to ectomycorrhizal fungi correlates with total belowground allocation in culture studies. Ecology 87:563–569. doi:10.1890/05-0755
Hobbie EA, Colpaert JV (2003) Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytol 157:115–126. doi:10.1046/j.1469-8137.2003.00657.x
Hobbie EA, Hobbie JE (2008) Natural abundance of 15 N in nitrogen-limited forests and tundra can estimate nitrogen cycling through mycorrhizal fungi: a review. Ecosystems (N Y, Print) 11:815–830. doi:10.1007/s10021-008-9159-7
Hobbie EA, Macko SA, Shugart HH (1999) Interpretation of nitrogen isotope signatures using the NIFTE model. Oecologia 120:405–415. doi:10.1007/s004420050873
Hobbie EA, Macko SA, Williams MT (2000) Correlations between foliar δ15N and nitrogen concentrations may indicate plant-mycorrhizal interactions. Oecologia 122:273–283. doi:10.1007/PL00008856
Hobbie EA, Jumpponen A, Trappe J (2005) Foliar and fungal 15 N:14 N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: testing analytical models. Oecologia 146:258–268. doi:10.1007/s00442-005-0208-z
Hobbie EA, Colpaert JV, White MW, Ouimette AP, Macko SA (2008) Nitrogen form, availability, and mycorrhizal colonization affect biomass and nitrogen isotope patterns in Pinus. Plant Soil 310:121–136. doi:10.1007/s11104-008-9637-x
Högberg P (1990) 15 N natural abundance as a possible marker of the ectomycorrhizal habit of trees in mixed African woodlands. New Phytol 115:483–486. doi:10.1111/j.1469-8137.1990.tb00474.x
Högberg P (1997) 15 N natural abundance in soil-plant systems. New Phytol 137:179–203. doi:10.1046/j.1469-8137.1997.00808.x
Kohzu A, Tateishi T, Yamada A, Koba K, Wada E (2000) Nitrogen isotope fractionation during nitrogen transport from ectomycorrhizal fungi, Suillus granulatus, to the host plant, Pinus densiflora. Soil Sci Plant Nutr 46:733–739
Lee LS, Alexander IJ, Watling R (1997) Ectomycorrhizas and putative ectomycorrhizal fungi of Shorea leprosula Miq. (Dipterocarpaceae). Mycorrhiza 7:63–81. doi:10.1007/s005720050165
Lilleskov EA, Hobbie EA, Fahey TJ (2002) Ectomycorrizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes. New Phytol 154:219–231. doi:10.1046/j.1469-8137.2002.00367.x
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620. doi:10.1111/j.1469-8137.2006.01936.x
Mayor JR, Schuur EAG, Henkel TW (2009) Elucidating the nutritional dynamics of fungi using stable isotopes. Ecol Lett 12:171–183. doi:10.1111/j.1461-0248.2008.01265.x
Moore D (1998) Fungal morphogenesis. Cambridge University Press, New York
Norvell LL (1998) Observations on development, morphology and biology in Phaeocollybia. Mycol Res 102:615–630. doi:10.1017/S0953756297005431
Ohenoja E, Metsänheimo K (1982) Phenology and fruiting body production of macrofungi in subarctic Finnish Lapland. Arctic and Alpine Mycology. In: Laursen GA, Ammirati JF (eds) The first international symposium on arcto-alpine mycology. University of Washington Press, Seattle, pp 390–409
Pardo LH, Templer PH, Goodale CL et al (2006) Regional assessment of N saturation using foliar and root δ15N. Biogeochemistry 80:143–171. doi:10.1007/s10533-006-9015-9
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162. doi:10.1016/S0169-5347(00)02098-X
Ruess RW, Hendrick RL, Burton AJ, Pregitzer KS, Svein-Bjornsson B, Allen MF, Maurer GE (2003) Coupling fine root dynamics with ecosystem carbon cycling in black spruce forests of interior Alaska. Ecol Monogr 73:643–662. doi:10.1890/02-4032
Schmidt S, Stewart GR (1997) Waterlogging and fire impacts on nitrogen availability and utilization in a subtropical wet heathland (wallum). Plant Cell Environ 20:1231–1241. doi:10.1046/j.1365-3040.1997.d01-20.x
Taylor AFS, Alexander IJ (2005) The ectomycorrhizal symbiosis: life in the real world. Mycologist 19:102–112. doi:10.1017/S0269915X05003034
Taylor AFS, Högbom L, Högberg M, Lyon AJE, Näsholm T, Högberg P (1997) Natural 15 N abundance in fruit bodies of ectomycorrhizal fungi from boreal forests. New Phytol 136:713–720. doi:10.1046/j.1469-8137.1997.00788.x
Taylor AFS, Martin F, Read DJ (2000) Fungal diversity in ecto-mycorrhizal communities of Norway spruce (Picea abies [L.] Karst.) and Beech (Fagus sylvatica L.) along north-south transects in Europe. In: Schulze E-D (ed) Carbon and nitrogen cycling in European forest ecosystems. Springer, Berlin, pp 343–365
Taylor AFS, Fransson PM, Högberg P, Högberg MN, Plamboeck AH (2003) Species level patterns in 13C and 15 N abundance of ectomycorrhizal and saprotrophic fungal sporocarps. New Phytol 159:757–774. doi:10.1046/j.1469-8137.2003.00838.x
Trudell SA, Edmonds RL (2004) Macrofungus communities correlate with moisture and nitrogen abundance in two old-growth conifer forests, Olympic National Park, Washington, USA. Can J Bot 82:781–800. doi:10.1139/b04-057
Trudell SA, Rygiewicz PT, Edmonds RL (2003) Nitrogen and carbon stable isotope abundances support the myco-heterotrophic nature and host-specificity of certain achlorophyllous plants. New Phytol 160:319–401. doi:10.1046/j.1469-8137.2003.00876.x
Trudell SA, Rygiewicz PT, Edmonds RL (2004) Patterns of nitrogen and carbon stable isotope ratios in macrofungi, plants and soils in two old-growth conifer forests. New Phytol 164:317–335. doi:10.1111/j.1469-8137.2004.01162.x
Unestam T, Sun Y-P (1995) Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza 5:301–311. doi:10.1007/BF00207402
Wallander H, Göransson H, Rosengren U (2004) Production, standing biomass and natural abundance of 15 N and 13C in ectomycorrhizal mycelia collected at different soil depths in two forest types. Oecologia 139:89–976. doi:10.1007/s00442-003-1477-z
Wallenda T, Kottke I (1998) Nitrogen deposition and ectomycorrhizas. New Phytol 139:169–187. doi:10.1046/j.1469-8137.1998.00176.x
Waring RH, Running SW (1998) Forest ecosystems: analysis at multiple scales. Academic, New York
Acknowledgments
This work was supported by NSF grants OPP-0612598, DEB-0614266, and DEB-147418 to Erik Hobbie. Comments of Andy Taylor, Steve Trudell, Roland Bol, Andy Ouimette, Thomas Kuyper, and two anonymous reviewers improved the manuscript and are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Thom W. Kuyper.
Rights and permissions
About this article
Cite this article
Hobbie, E.A., Agerer, R. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 327, 71–83 (2010). https://doi.org/10.1007/s11104-009-0032-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-0032-z