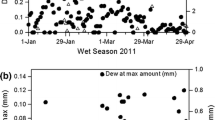
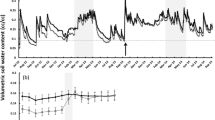
Abstract
Semiarid areas in the US have realized extensive and persistent exotic plant invasions. Exotics may succeed in arid regions by extracting soil water at different times or from different depths than native plants, but little data is available to test this hypothesis. Using estimates of root mass, gravimetric soil water, soil-water potential, and stable isotope ratios in soil and plant tissues, we determined water-use patterns of exotic and native plant species in exotic- and native-dominated communities in Washington State, USA. Exotic and native communities both extracted 12 ± 2 cm of water from the top 120 cm of soil during the growing season. Exotic communities, however, shifted the timing of water use by extracting surface (0–15 cm) soil water early in the growing season (i.e., April to May) before native plants were active, and by extracting deep (0–120 cm) soil water late in the growing season (i.e., June to July) after natives had undergone seasonal senescence. We found that δ 18O values of water in exotic annuals (e.g., −11.8 ± 0.4 ‰ for Bromus tectorum L.) were similar to δ 18O values of surface soil water (e.g., −13.3 ± 1.4 ‰ at −15 cm) suggesting that transpiration by these species explained early season, surface water use in exotic communities. We also found that δ 18O values of water in taprooted exotics (e.g., −17.4 ± 0.3 ‰ for Centaurea diffusa Lam.) were similar to δ 18O values of deep soil water (e.g., −18.4 ± 0.1 ‰ at −120 cm) suggesting that transpiration by these species explained late season, deep water use. The combination of early-season, shallow water-use by exotic winter-actives and late-season, deep water-use by taprooted perennials potentially explains how exotic communities resist establishment of native species that largely extracted soil water only in the middle of the growing season (i.e., May to June). Early season irrigation or the planting of natives with established root systems may allow native plant restoration.




Similar content being viewed by others
References
Bonet A, Pausas JG (2004) Species richness and cover along a 60-year chronosequence in old-fields of southeastern Spain. Plant Ecol 174:257–270
Corbin JD, D’Antonio CM (2004) Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology 85:1273–1283
Corbin JD, Thomsen MA, Dawson TE, D’Antonio CM (2005) Summer water use by California coastal prairie grasses: fog, drought, and community composition. Oecologia 145:511–521
Dawson TE, Ehleringer JR (1991) Streamside trees that do not use stream water. Nature 350:335–337
DiTomaso JM (2000) Invasive weeds in rangelands: species, impacts, and management. Weed Sci 48:255–265
Dyer AR, Rice KJ (1999) Effects of competition on resource availability and growth of a California bunchgrass. Ecology 80:2697–2710
Enloe SF, DiTomaso JM, Orloff SB, Drake DJ (2004) Soil water dynamics differ among rangeland plant communities dominated by yellow starthistle (Centaurea solstitialis), annual grasses, or perennial grasses. Weed Sci 52:929–935
Ewe SML, Sternberg LDL (2002) Seasonal water-use by the invasive exotic, Schinus terebinthifolius, in native and disturbed communities. Oecologia 133:441–448
Foster BL, Tilman D (2000) Dynamic and static views of succession: testing the descriptive power of the chronosequence approach. Plant Ecol 146:1–10
Gazis C, Feng XH (2004) A stable isotope study of soil water: evidence for mixing and preferential flow paths. Geoderma 119:97–111
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed), Methods of soil analysis, Part 1. Physical and Mineralogical Methods. Soil Science Society of America, Madison, WI, pp383–411
Heuscher SA, Brandt CC, Jardine PM (2005) Using soil physical and chemical properties to estimate bulk density. Soil Sci Soc Am J 69:51–56
Holmes TH, Rice KJ (1996) Patterns of growth and soil-water utilization in some exotic annuals and native perennial bunchgrasses of California. Ann Bot 78:233–243
Kendall C, Coplen TB (2001) Distribution of oxygen−18 and deuterium in river waters across the United States. Hydrol Processes 15:1363–1393
Kulmatiski A (In press) Exotic plants establish persistent communities. Plant Ecol. DOI: 10.10 07/s112 58–006-914 0-5
Leffler AJ, Peek MS, Ryel RJ, Ivans CY, Caldwell MM (2005) Hydraulic redistribution through the root systems of senesced plants. Ecology 86:633–642
Lenfesty CD (1980) Soil survey of Okanogan county area. National Cooperative Soil Survey, Washington
Ludwig F, Dawson TE, Prins HH T, Berendse F, de Kroon H (2004) Below-ground competition between trees and grasses may overwhelm the facilitative effects of hydraulic lift. Ecol Lett 7:623–631
Marler MJ, Zabinski CA, Wojtowicz T, Callaway RM (1999) Mycorrhizae and fine root dynamics of Centaurea maculosa and native bunchgrasses in western Montana. Northwest Sci 73:217–224
Norton JB, Monaco TA, Norton JM, Johnson DA, Jones TA (2004) Soil morphology and organic matter dynamics under cheatgrass and sagebrush-steppe plant communities. J Arid Environ 57:445–466
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Roche BF, Roche CT (1998) Diffuse Knapweed. In: Sheley RL, Petroff JK (eds) Biology and management of noxious rangeland weeds. Oregon State University Press, Corvallis, pp217–230
Saxton KE, Rawls WJ, Romberger JS, Papendick RI (1986) Estimating generalized soil water characteristics from texture. Soil Sci Soc Am J 50:1031–1036
Sheley RL, Jacobs JS, Carpinelli MF (1998) Distribution, biology, and management of diffuse knapweed (Centaurea diffusa) and spotted knapweed (Centaurea maculosa). Weed Technol 12:353–362
Sheley RL, Larson LL (1994) Comparative life-history of cheatgrass and yellow starthistle- observation. J Range Manage 47:450–456
Sheley RL, Petroff JK (1998) Biology and management of noxious rangeland weeds. Oregon State University Press, Corvallis
Stratton LC, Goldstein G, Meinzer FC (2000) Temporal and spatial partitioning of water resources among eight woody species in a Hawaiian dry forest. Oecologia 124:309–317
Stylinski CD, Allen EB (1999) Lack of native species recovery following severe exotic disturbance in southern Californian shrublands. J Appl Ecol 36:544–554
Takahashi K (1998) Oxygen isotope ratios between soil water and stem water of trees in pot experiments. Ecol Res 13:1–5
Webb EA, Longstaffe FJ (2003) The relationship between phytolith- and plant-water delta O-18 values in grasses. Geochim Cosmochim Acta 67:1437–1449
White JWC, Cook ER, Lawrence JR, Broecker WS (1985) The deuterium to hydrogen ratios of sap in trees: implications for water sources and tree ring deuterium to hydrogen ratios. Geochim Cosmochim Acta 49:237–246
Williams DG, Ehleringer JR (2000) Intra- and interspecific variation for summer precipitation use in pinyon-juniper woodlands. Ecol Monogr 70:517–537
Yamanaka T, Yonetani T (1999) Dynamics of the evaporation zone in dry sandy soils. J Hydrol 217:135–148
Acknowledgements
This research was funded by USDA - NRI (# 35320–13473), the Utah State Agricultural Experimental Station, the Switzer Foundation, and Sigma Xi. We thank the WA Department of Wildlife, especially J. Mountjoy, Rainier Seeds Inc., X. Feng, G. P. Kyle for field assistance, and P. Attaphongse, and L. Prichard for laboratory assistance. We also thank S. Durham for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulmatiski, A., Beard, K.H. & Stark, J.M. Exotic plant communities shift water-use timing in a shrub-steppe ecosystem. Plant Soil 288, 271–284 (2006). https://doi.org/10.1007/s11104-006-9115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9115-2