Abstract
The essence of the inversion concept of the origin of life can be narrowed down to the following theses: 1) thermodynamic inversion is the key transformation of prebiotic microsystems leading to their transition into primary forms of life; 2) this transformation might occur only in the microsystems oscillating around the bifurcation point under far-from-equilibrium conditions. The transformation consists in the inversion of the balance “free energy contribution / entropy contribution”, from negative to positive values. At the inversion moment the microsystem radically reorganizes in accordance with the new negentropy (i.e. biological) way of organization. According to this approach, the origin-of-life process on the early Earth took place in the fluctuating hydrothermal medium. The process occurred in two successive stages: a) spontaneous self-assembly of initial three-dimensional prebiotic microsystems composed mainly of hydrocarbons, lipids and simple amino acids, or their precursors, within the temperature interval of 100–300°C (prebiotic stage); b) non-spontaneous synthesis of sugars, ATP and nucleic acids started at the inversion moment under the temperature 70–100°C (biotic stage). Macro- and microfluctuations of thermodynamic and physico-chemical parameters able to sustain this way of chemical conversion have been detected in several contemporary hydrothermal systems. A minimal self-sufficient unit of life on the early Earth was a community of simplest microorganisms (not a separate microorganism).
Similar content being viewed by others
Introduction
The origin-of-life problem can be divided into two coupled questions; the core of them was stated by Ehrenfreund et al (2006):
-
1.
Which prebiotic resources can potentially form building blocks, and how can they be subsequently transformed into containers, metabolic reaction network, and informational polymers?
-
2.
How can these three components assemble into a protocell that would satisfy minimal requirements for a living system?
Investigators of this problem have developed their research from prebiotic chemistry to the primary organization of living systems. Meanwhile, the second question is closely connected with a thermodynamic aspect of the biological organization. The key thermodynamic notions, like free energy and entropy, seem to be universal and applicable to various habitable worlds in the Universe. In fact, this question corresponds to the 2nd scientific goal of Astrobiology: “Determine the general principles governing the organization of matter into living systems” (Morrison 2001). At the same time, the first question is actually considered on the basis of biochemistry of the Earth. However, biochemistries of extraterrestrial habitable worlds may differ from the terrestrial ones. So, it would be reasonable to consider the origin-of-life problem in the reverse order: from a general method of biological organization to prebiotic chemistry and early biochemistry on the Earth. It is such an attempt that has been made by the author in this article.
Thermodynamic Aspect of the Origin of Life
Entropy, Free Energy and Information
Entropy (S), free energy (F) and information (I) are the key characteristics determining the macro-state of a system and its evolution. Entropy (S) is an integrated characteristic: it doesn’t reduce to the sum of the system’s constituents, which can be described by strict equations only. When describing self-organization in open systems the entropy notion serves as both the measure of energy value (the more entropy, the less useable energy value) and the measure of disorganization (the more entropy, the higher disorganization) (Ebeling et al 1990; Lin 1996). High-value energy can be defined in terms of free energy. Free energy is a part of the system entire internal energy, which can be converted into any kind of work (mechanical, chemical, etc.). It is implied that the term free energy used in this paper corresponds to Gibbs energy. Nevertheless, the author uses this term in a rather common sense allowing him to compare thermodynamic processes in biological and non-biological systems; for this reason, free energy is denoted as F (not G). The remained internal low-value energy (bound energy) cannot be converted into work
A smaller part of entropy transmission is connected with informational processes. The entry of information (I) reduces disorganization in a system. From a physical point of view, information can be understood as the value which reduces uncertainty in the state of a system. The transfer of information in systems is always related with a corresponding transfer of entropy and energy. The information flow represents a special case of entropy transfer between two systems. Informational entropy is the form of entropy directly connected with informational processes (Ebeling et al 1990; Feistel and Ebeling 2011). So, entropy, free energy and information are interrelated values. Generally, contributions to free energy and information decrease entropy and vice versa.
Thermodynamic Trend of Biological Evolution
The general thermodynamic trend of biological evolution can be expressed in terms of entropy, free energy and information changes. Free energy accumulates in the course of the biosphere’s evolution (f.i.: Vernadsky 1980; de Duve 2002). Sometimes this tendency is indicated as the accumulation of high energy substance (Gladyshev 1995). The entropy change, in the course of biological evolution, takes place in the field of negative values, as the export of entropy from biological systems prevails over its internal production. In this way negative entropy (negentropy) increases and positive entropy (i.e. proper entropy) decreases (Ebeling et al. 1990; Elitzur 2002; Polishchuk 2002; etc.). This thermodynamic trend corresponds to the living system’s ability to concentrate free energy and information, which is considered by many scientists to be one of the fundamental biological properties, as mentioned in the article by Kompanichenko (2008). This ability allows biological systems to have an excess of free energy and information with respect to the environment and maintain corresponding positive F and I gradients.
The thermodynamic trend of biological evolution is a phenomenon which is not characteristic of any non-biological natural system. Only a biological system is able to extract free energy and information from the environment at the expense of its own activity. Some natural systems, for instance, volcanoes or stars, can accumulate free energy and preserve the excess. However, these two types of natural systems enrich the surroundings with energy, while a biological system is active extracting energy out of the medium (Kompanichenko 2003). A living organism is able to be active with respect to the environment, because of the entropy pump inside (Ebeling et al. 1990; Feistel and Ebeling 2011).
Inversion of Thermodynamic Trend: Start of Biological Evolution
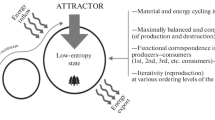
As stated above, biological evolution proceeds in the thermodynamic direction opposite to the evolution of non-biological natural systems (Fig. 1, top). This direction means a concentration (accumulation) of free energy and information correlated with the entropy moving into the area of negative values. Therefore, the beginning of biological evolution implies the inversion in the tendency of free energy change from dissipation to continuous increase, as well as the inversion in the tendency of information change (from spontaneous loss to enduring accumulation) and entropy (from positive to negative values). The change of entropy is a general expression of the thermodynamic inversion. As entropy serves as a measure of both energy value and disorganization, thermodynamic inversion can be itemized through a change of the balances “free energy contribution / entropy contribution (Fc / Sc)”, and “contribution of information / contribution of informational entropy (Ic / Sic). How might the inversion of thermodynamic trend occur during the transition from prebiotic microsystems to primary living units (Fig. 1, bottom)? The author’s answer to this question will be formulated in “Formation of Initial Living Units (Probionts) ” and illustrated in Fig. 8.
Thermodynamic inversion can be expressed as follows:
F+ is the contribution of free energy into a system, both at the expense of its internal production and extraction of energy from the outside world; F− represents loss of free energy in a non-biological or biological system, both at the expense of internal devaluation and dissipation in the outside world; n-bs – non-biological systems; bs represents biological systems.
I+ is the input of information into a system; I− is the loss of information in a system;
S is entropy, S+ is contribution of (positive) entropy into a system, S− is contribution of negentropy into a system (both at the expense of internal production and extraction from the outside world).
Later in the text balances Fc / Sc and Ic / Sic will be integrated into a common balance Fc + Ic / Sc (contribution of free energy and information / contribution of entropy). The prevalence of contribution of free energy and information over the entropy contribution allows the existence of a biological system. The biological evolution negentropy trend is connected with the positive balance Fc + Ic / Sc. Nevertheless, the balance Fc + Ic / Sc is relative: absolute equality in this ratio is unattainable, as far as free energy and information are measured in different units. It follows that a certain level of uncertainty exists close to the inversion point Fc + Ic ≈ Sc. This field admits the availability of prevalent entropy contribution in one part of a heterogeneous system and the contribution of free energy and information prevalence in its other part. In fact, this field of uncertainty represents a (positive) entropy barrier the prebiotic microsystem must overcome to transform into a living unit. In particular, Strazewski (2007) wrote about a high entropy penalty for the formation of polynucleic acids ordered association. The penalty can be considered as one of this barrier’s constituents. Another constituent is connected with the entropy pump within a prebiotic microsystem at the inversion moment. To start the entropy pump, the entropy export in the microsystem must exceed a certain critical value.
Thermodynamic inversion reverses the position of the chemical system from passive to active with respect to the environment. At the point in time when prebiotic organic microsystems transform into initial biotic units they arise as “centers of activity” in the medium. In this way, the transformed microsystems acquire the following four key biological properties: 1) ability to concentrate free energy and information (by means of active extraction from the environment); 2) ability to exhibit an intensified counteraction to external influences; 3) expedient behavior; 4) regular self-renovation at various levels, including self-reproduction (Kompanichenko 2008, 2012a). These key properties integrate 31 fundamental properties of biological systems defined by 73 competent scientists (Palyi et al. 2002).
General Mechanism of Thermodynamic Inversion
This section is a compact description of the general mechanism of thermodynamic inversion in prebiotic microsystems (it will be considered in detail in the following sections). The mechanism includes three stages: 1) bifurcation, 2) (relative) stabilization, 3) inversion (Kompanichenko 2008, 2009a). The originality of the author’s approach to the origin of life can be expressed in the following statement: it is thermodynamic inversion that is responsible for the transition of prebiotic microsystems into living units (3rd stage), and this transformation might occur only in the microsystems oscillating around the bifurcation point, under far-from-equilibrium conditions (1st and 2nd stages).
The universal scheme of bifurcation of a system is as follows: stable existence of a system → rise of instability through powerful fluctuations → the highest point of instability (point of bifurcation, or critical point), radical change of the system’s structure → choice of a new path of the development → subsequent stage of its stable existence (Fig. 2a). At the bifurcation point a system undergoes numerous accidental changes influencing its further development. This is the reason why the system’s potential paths of development bifurcate at the moment of its highest instability. Finally, the system chooses one of the permissible ways subdivided into two principal trends: (1) complication through self-organization (Trend A), (2) simplification and degradation (Trend B), till its complete destruction (Trend B’). The reverse of external conditions causes the transition of a system into the initial state, in accordance with Trend C (Fig. 2b).
The principal scheme of bifurcation of a natural system under far-from-equilibrium conditions. ‘a’—direct transition from the initial stable state into one of the permissible advanced stable states, due to the changed conditions in the outside world;. ‘b’—direct and reverse transitions (in case oscillating conditions in the outside world). A—trend to the advanced higher-organized state; B—trend to the advanced lower-organized state; B’—trend to complete the destruction; C’ and C”—reverse trend to the approximately initial state (modified from Kompanichenko 2008)
A drastic transition of the prebiotic microsystem (its composition will be discussed later) over the entropy barrier is possible through a super-critical negentropy (constructive) impulse. The impulse can be generated in the microsystem oscillating around the bifurcation point between two attractors—the initial and the advanced stable states. First a prebiotic microsystem loses its initial stable state, due to a significant change of thermodynamic and/or physico-chemical parameters in the outside world, and tends to transit into a new stable state, through the unstable point of bifurcation (Fig. 3). Bifurcation of chemical systems under far-from-equilibrium conditions is well-explored in the framework of the theory of dissipative structures and synergetics (Haken 1978, 2003; Nicolis and Prigogine 1977; Prigogine and Stengers 1984; Ebeling et al. 1990; Feistel and Ebeling 2011). A rare case of balanced oscillations of a prebiotic microsystem around the bifurcation point is conducive to the origin-of-life process. As a result, such a microsystem exists in the oscillating regime, its internal structure bifurcated (Fig. 3). This type of system was called bi-state (Kompanichenko 2004). A bistate system can be considered as a specific type of bistable system. It is characterized by its paradoxical tendencies to simultaneous separation and integration, because of balanced oscillations between two attractors (the initial and new stable states). The properties of a bistate chemical system were theoretically substantiated in the previous work by the author (Kompanichenko 2008).
Attraction of the initial equilibrium state I and the advanced equilibrium state II to the point of bifurcation. Dotted circles—areas of relative stability of the nonequilibrium states I’ and II’ that are in a balance. The grey field is the area of ‘interpenetrating’ of the nonequilibrium states I’ and II’ around the point of bifurcation
Due to the oscillating character of the bistate microsystem existence, the contribution of free energy might, from time to time, prevail over the contribution of entropy, bringing it into the thermodynamic niche of the initial life (Fig. 4, I-Ia). This transition is provided by the super-critical negentropy impulse generated in the prebiotic microsystem. Such an impulse could emerge due to the preceding free energy pumping into a system (Ebeling et al. 1990). Under favorable conditions the oscillating organic microsystem might accumulate a surplus of free energy bound in high energy compounds. A significant external action (stress) could lead to a fast release of the energy surplus that generated a powerful negentropy impulse, followed by a temporal transition of the oscillating microsystem into the thermodynamic niche of the initial life. Either the ascending or the descending thermodynamic trend is possible for the microsystem’s further evolution. If the microsystem develops what appear to be biological properties, it follows the ascending trend and transforms into the initial living unit—a probiont (Fig. 4, II). Its inability for a further development results in the descending trend, going down below the entropy barrier.
Oscillation of the balance “free energy contribution / entropy contribution” in a bistate prebiotic microsystem (I) and inversion of the balance into primary living unit—probiont (II) (modified from Kompanichenko 2008)
The term anastrophe, meaning a drastic constructive transformation of a biological system, can also be used to designate the considered super-critical negentropy impulse. The term was introduced by H. Baltscheffsky (1997), who described some anastrophes in the early evolution of biological energy conversion. According to the inversion concept, this negentropy impulse was the initial greatest anastrophe, which had laid the foundation of life on the Earth.
Appropriate Medium for the Origin of Life
Consequences of the Inversion Approach Referring to the Medium for the Origin of Life
The ocean and hydrothermal systems are the two media which are usually considered to be suitable for the origin of life. They both satisfy three well-known conditions for the origin of life: liquid environs, the availability of organic matter and a source of energy. However, the inversion approach supplements it with the forth necessary condition: the availability of changes in the medium, including irregular and regular fluctuations of thermodynamic and physico-chemical parameters. The condition was necessary from both biological and physical points of view.
The ability to exhibit an intensified counteraction to external actions (2nd key biological property) implies the availability of changes in the outside world at the moment of primary biological systems formation. An arising living unit must undergo external influences to have an opportunity for realizing this property and extracting surplus energy from the environment. So, the non-oscillatory environment doesn’t offer the initial biological system any chance for concentrating free energy and sustaining its negentropy method of organization. Besides, the necessity of stress (i.e. external influences) for a living organism is stated in the theory of stress: the absence of stress means the absence of life (Selye 1974).
From the physical point of view, a prebiotic microsystem acquires specific properties only during bifurcation. These are the properties underlying the living processes: high heterogeneity with intensive counter processes along and against gradients; continuous fluctuations and re-arrangement of molecules; integrity through cooperative processes; incessant exchange by matter and energy with the outside world (Nicolis and Prigogine 1977; Haken 1978; Ebeling et al. 1990; Kompanichenko 2008). Life is impossible if it is devoid of these properties, but a chemical system loses them as soon as the bifurcation is over and the system converts into a new stable state. Under favorable external oscillations a prebiotic microsystem oscillates around the point of bifurcation and in this way prolongs its bifurcational state. However, in the non-oscillatory conditions of the medium the microsystem should irreversibly get into a new stable state.
It is supposed that thermodynamic and physico-chemical fluctuations suitable for the origin-of-life medium should take place at least at two levels—irregular macrofluctuations and regular microfluctuations. Macrofluctuations with significant amplitudes facilitate the turning of a prebiotic microsystem from the stable to the unstable state (values from 1 to 3, Fig. 2). These changes, indicated as macro-fluctuations, should have an irregular character to prevent a microsystem from returning to an initial stable state. The availability of regular microfluctuations, or smooth oscillations, is necessary to sustain the intermediate position of a prebiotic microsystem between two attractors—the initial and the new stable states. A chemical system, when at the bifurcation point, is unstable in principle. Therefore, it is only the external oscillations in the optimal regime that could maintain balanced oscillations of a prebiotic microsystem around the bifurcation point. These changes should be quite regular, with small amplitude of the relevant parameter(s) (between the values of 2 and 3, Fig. 2). This combination of weak regular oscillations and significant irregular fluctuations (noise) is favorable for the appearance of stochastic resonance that could provide the prebiotic bistate microsystem with an additional impulse to surmount the entropy barrier and occupy the thermodynamic niche of the initial life (Fig. 4).
To develop the ability for the intensified and expedient response to external changes, a probiont must be exposed to multiple effects from the environment during its cycle of existence—from the initial fission (birth) to the last one (death). In other words, in the environment, both high-frequency thermodynamic and physico-chemical oscillations with periods much shorter than the probionts lifetime should be available. An average lifecycle of the thermophilic prokaryotes at the root of the Phylogenetic Tree are of the order of several hours. Consequently, the appropriate periods of fluctuations in the environment should vary from a few minutes to approximately one hour.
Thus, the following general characteristics of thermodynamic and physico-chemical fluctuations in the maternal medium for the origin of life can be outlined:
-
1.
The fluctuations should vary and display themselves at least at two levels: irregular macrofluctuations with considerable amplitudes and regular microfluctuations with small amplitudes.
-
2.
The high frequency fluctuations (with periods less than one hour) should be available in the original environment.
Defining the Appropriate Medium for the Origin of Life
The scale of thermodynamic and physico-chemical fluctuations in aqueous media depends on the scale of corresponding gradients. From this point of view, hydrothermal systems possess far more potency for generating macrofluctuations, than the ocean (beyond hot vents on its bottom). For instance, in the hydrothermal media the salinity varies within the range <1–500 g/l, pH 1–12, the temperature 30–400°C. The scale of fluctuations in hydrothermal systems is diverse and ranges from very low to extraordinary high. Macrofluctuations in contemporary hydrothermal systems are initiated by earthquakes, tectonic dislocations, volcanic eruptions, and internal explosive events. The oceans on the Earth have very low gradients (for instance, the minimal and maximal values of the salinity are 30 and 38 g/l, pH 8.0 and 8.4), correspondingly. The low gradients predetermine small amplitudes of fluctuations of thermodynamic and physico-chemical parameters in the ocean.
To characterize the regime of thermodynamic fluctuations in natural hydrothermal systems, we have mathematically processed the monitoring database on fluid pressure and temperature in boreholes of the Mutnovsky and Pauzhetsky thermal fields in the Kamchatka peninsula (Kompanichenko 2009a). The record of pressure changes in the Mutnovsky field borehole № 30 displays both irregular macrofluctuations and sufficiently regular microoscillations. The record fragments are shown in Fig. 5a–b. The period of pressure microoscillations of the water-steam mixture is about 20 min (Fig. 5b). The average correlation coefficient between pressure and temperature in the Mutnovsky field estimated for 12 boreholes is very high - 0.96. Thus, the described macro- and micro fluctuations refer to both thermodynamic parameters. The periods of pressure microfluctuations in 11 boreholes of the Pauzhetsky field ranges from 10 to 60 min. Nevertheless, in 2 boreholes a short (1 day) monitoring has shown the absence of pressure fluctuations. The amplitudes of macrofluctuations in the Mutnovsky and Pauzhetsky fields may reach 1–2 bars; and the amplitudes of microfluctuations occur within the interval of 0.1–0.3 bars. According to Kralj and Kralj (2000), microoscillations of hot water pressure were also detected in the Mura hydrothermal field boreholes, Slovenia (Fig. 5c–d). In this field the periods of pressure and temperature oscillations are the same (about 70 min) but correlation between them is negative (Fig. 6a). Kralj and Kralj (2000) explored in detail a change of chemical parameters during one 70-minute cycle of thermodynamic oscillations by using high-frequency sampling. It was revealed that the temperature change positively corresponds with the change of many chemical parameters (Fig. 6a–d). Concentrations of Na+, K+, Ca2+, HCO −3 , SO 2−4 , Cl−, Br− and pH follow the temperature peak with the 15-minutes delay. Weak concentrations peaks of I−, F−, CO2 also correlate with the temperature peak. Only the Mg2+ and NH +4 contents do not display a definite tendency. Summarizing all theoretically substantiated characteristics of fluctuations in the origin-of-life medium, in hydrothermal systems it has been detected: a) high-amplitude (up to 1–2 bars) irregular macrofluctuations of thermodynamic parameters; b) low-amplitude quite regular microoscillations of pressure (amplitudes 0.1–0.3 bars), temperature (0.5–1°C) and concentrations of most chemical components; c) periods of microoscillations occur within the range of 10–70 min.
Oscillations of pressure of water and water-steam mixture in hydrothermal systems: examples of the records. ‘a’, ‘b’: oscillations of pressure in well № 30 at a depth of 950 meters, Mutnovsky hydrothermal field (Kamchatka), record in July, 2006 (modified from Kompanichenko 2009a). ‘c’, ‘d’: oscillations of pressure in well Sob-1, Mura geothermal basin (Slovenia), record in November, 1997 (Kralj and Kralj 2000)
Correlative time-dependent variations of thermodynamic and physico-chemical parameters in hot water during one cycle of microoscillations (70-min time interval) in well-head Sob-1, Mura geothermal basin (Slovenia), record in November, 1997 ‘a’—change of pressure (P) and temperature (T); ‘b-d’—variations in abundances of various ions, trace elements and undissolved gases: Cl−, F−, Br−, I−.(b), Na+, HCO −3 , CO2 (c), K+, Ca2+, Mg2+, NH +4 , SO 2−4 (d) (Kralj and Kralj 2000)
So, hydrothermal systems satisfy the 4th necessary condition for the origin of life, substantiated within the framework of the inversion approach: thermodynamic and/or physico-chemical fluctuations in the maternal medium. The incessant fluctuations in hydrothermal systems are maintained by counteractions between rising hydrodynamic and descending lithostatic pressures. The ocean (but not hydrothermal discharges on the ocean floor) is a far less appropriate medium for the origin of life, as this medium is characterized by the decreasing hydrostatic pressure, and the absence of counteracting pressures. Correspondingly, the scale of gradients and fluctuations of thermodynamic and physico-chemical parameters in the ocean is very low. Besides, the minimal period of temperature oscillations in the terrestrial oceans is 24 h (day-night), which exceeds the lifetime of thermophilic prokaryotes. Such long-term oscillations do not allow the transformation of prebiotic microsystems into living probionts, according to the inversion approach. In this connection, the author, as well as many other scientists (Corliss et al. 1981; Pace 1991; Washington 2000; Schwartzman and Lineweaver 2004; Holm and Andersson 2005; Russell et al. 2005; Baaske et al 2007; Feistel and Ebeling 2011) considers hydrothermal systems to be the most probable medium for the origin of life on the early Earth. In the context of the potential cradle of life the following geological settings can be considered: hydrothermal systems in the upper part of the Earth’s crust; high temperature fluid discharges on the ocean floor; littoral discharges of hot water (with both geothermal energy and sunlight available); hydrothermal discharges into the continental ground-water aquifers. The changeability of parameters in submarine vents is usually lower than in terrestrial hydrothermal systems, but their fluctuation amplitude tends to increase as a result of earthquakes or volcanic events.
The range of temperature from 70 to approximately 100°C seems to be most favorable for the origin of probionts and their communities (though, the dispersion for prebiotic microsystems existence can be much wider). A temperature higher than 100°C could hamper condensation of water in the Hadean Ocean and upper aquifers of groundwater systems. In addition, the nucleotide chains of probionts cannot be synthesized in Vitro at a temperature lower than 90°C. Estimations of the temperature of water in the Archean ocean have ranged from +70–80°C (Knauth and Lowe 2003; Robert and Chaussidon 2006) to the temperature as it is today (Shields and Kasting 2007).
Organic Matter in Natural and Simulated Hydrothermal Medium
The next aspect in considering hydrothermal systems as a potential cradle of life deals with the organic substance availability. It is important to determine the organic compounds actually available in the systems, and those on the basis of model experiments. A significant part of the organics in contemporary hydrothermal systems, especially in inhabited discharges with the temperature below 100–110°C, has a biogenic origin (destruction of microorganisms, extraction of buried organics from host rocks). The abiogenic part could be synthesized in the hydrothermal media, or be injected into the subsurface water circulation from other sources (synthesis during volcanic eruptions and lightning in the atmosphere), and it could also be delivered with comets and meteorites of the Murchison type (as considered by Markhinin and Podkletnov 1977; Basiuk and Navarro-Gonzalez 1996; Deamer 1985; Yuen and Kvenvolden 1973). Apparently, it is very difficult to determine the ratio of biogenic and abiogenic organics in natural hot solutions, taking into consideration possible geochemical and biotic transformations. Since the organic substance composition in the Archean hydrothermal systems differed from the contemporary one, the early Earth being lifeless, we can only define a probable set of organic compounds in ancient hydrothermal systems, by integrating the organic content of contemporary natural environments with the results of model experiments in aqueous high temperature conditions, such as in reviews by Simoneit 2004 andHolm and Andersson 2005).
Organic Matter in Contemporary Hydrothermal Systems
Hydrocarbons and lipid compounds are wide-spread in hydrothermal fluid. In particular, linear saturated hydrocarbons are revealed in fluid of Rainbow field on the Mid-Atlantic Ridge (Holm and Charlou 2001). The chain length of these hydrocarbons consists of 16–29 carbon atoms and it can be compared to the gabbros fluid inclusions in Southwest Indian Ridge that showed the presence of C2-C5 hydrocarbons (Kelley 1996). The typical hydrothermal petroleum from Guaymas basin has an intermediate content of n-alkanes (18 %) with a relatively normal content of iso-, anteiso-, isoprenoid and naphthenic hydrocarbons (82 %), comparable to crude oils (Didyk and Simoneit 1990). Various hydrothermal minerals deposited inside the Trans-Atlantic Geotraverse hydrothermal field on the Mid-Atlantic Ridge contain trace amounts of C10-C22 hydrothermal petroleum, consisting of n-alkanes and PAH (Simoneit 1993). The composition of lipid components in hydrothermal sulfide deposits from the Rainbow vent field, Mid-Atlantic Ridge, was explored by B. Simoneit et al. (2004). They detected 64 fatty acids, including five 10-Me branched fatty acids and eleven hydroxy fatty acids. We explored ten continental hydrothermal fields in the Russian Far East. According to the obtained data, the sterile condensate of water-steam mixtures (temperature 108–175°C) from deep boreholes contains 20 organic compounds, which belong to 5 homologous series: aromatic hydrocarbons, n-alkanes, aldehydes, ketones and alcohols (Kompanichenko 2009c). In addition, Cl-hydrocarbons (Cl-alkanes), ethers (dietoxyalkanes, dimetoxyalkanes) and lipid precursors (fatty acids, methyl ethers of fatty acids) were detected in the lower temperature solutions (T = 55–99°C) from hot springs inhabited by thermophiles and hyperthermophiles. Free amino acids are not abundant components in natural hot solutions; however, sometimes their presence is proved. Thus, glycine was revealed in the sterile condensate of water-steam mixture from a deep borehole in the Kamchatka peninsula (Mukhin et al. 1979).
The gas phase of hydrothermal systems can also be rich in organics. For instance, Isidorov et al. (1992) explored volatile organics in 7 steam-gas outflows of several volcanoes and thermal fields in Kamchatka. They detected about 60 organic compounds belonging to 13 homologous series (n-alkanes, cyclic alkanes, alkenes, aromatics, alcohols, ketones, esters, Cl-alkanes, etc.). One of the basic gases in hydrothermal fluids is methane. It is prevalent (up to 90 %) in the gas phase in hydrothermal systems of the methane geochemical type. Condensed phosphates are emitted in volcanic fumaroles (Yamagata et al. 1991). As is well known, condensed phosphates are the universal biological currency (mainly in the ATP form). A distribution of chemical components between the liquid and steam-gas phases in a hydrothermal system depends on variations of thermodynamic and physico-chemical parameters. Thus, the crack opening due to tectonic processes may result in a fall of pressure with the following ebullition of the liquid phase and redistribution of the compounds.
Biologically Important Organic Matter in Simulated Hydrothermal Conditions
There exists a great variety of geochemical hydrothermal environments. Black smokers situated along basaltic spreading centers are often characterized by a very high temperature (300–400°C), hydrostatic pressure (up to 400–500 bars) and acidity (pH 3–4). Off-axis submarine discharges can be alkaline (pH ≈ 11) and lower-temperature (about 100°C) (Russell 2003). Continental thermal springs are also very diverse, from the thermodynamic and physico-chemical points of view. In fact, all these natural conditions have been simulated in the experiments on prebiotic chemistry modeling the hydrothermal environment.
The abiotic synthesis of hydrocarbons is possible under the conditions simulating hydrothermal vents at the oceanic spreading center, in particular through CO2 reduction during the process of serpentinization. The Fischer-Tropsch type (FTT) reaction is one of the possible ways to produce hydrocarbons and oxy compounds from carbon monoxide and carbon dioxide. Although the yield of light hydrocarbons in a hot liquid phase can be limited, it is enhanced in the presence of iron- and chromium-bearing minerals (Berndt et al. 1996; McCollom and Seewald 2001). Thermo catalytic (analogous to FTT) reactions are more efficient to produce abiotic organics under aqueous conditions (McCollom et al. 1999; Rushdi and Simoneit 2001). The optimum yield of products (up to 5 % carbon fixation) was obtained at 200°C. In the synthesis products from experiments conducted above 150°C the homologous series of lipids compounds predominated, i.e., straight chain n-alkanols, n-alkanoic acid, alkyl formats, n-alkanes, etc. At temperatures above 300°C synthesis competes with cracking and reforming reactions (Simoneit 2004). Synthesis of fatty acids proceeds in the conditions modeling the hydrothermal vent system (Shock et al. 1998). Under supercritical conditions of water abiotic organic reactions could proceed as well. Both oxidative and reductive reactions can easily be carried out in modified supercritical fluids (Holm and Andersson 2005).
Synthesis of amino acids under hydrothermal conditions has already been reported (Hennet et al. 1992; Marshall. 1994). Studies at high temperatures and pressure in the laboratory have revealed a number of reactions that proceed rapidly in hydrothermal fluids, including the Strecker synthesis of amino acids (Holm and Andersson 2005). However, the behavior of various amino acids in simulated hydrothermal environments can be different, and it depends on temperature, pressure and mineral assemblages buffering pH, and redox conditions. Thus, at 250°C and 265 bar aspartic acid, serine and leucine decomposed rapidly, but the concentrations of alanine and glycine remained relatively stable or even increased. It is remarkable that amino acids were extremely sensitive to the existing redox conditions (Bernhardt et al. 1984; Miller and Bada 1988). The opposite trends to decomposition and recovery of amino acids were revealed in the experiments. In particular, the rates of amino acid (aspartic acid, serine, leucine and alanine) decomposition were evaluated at 200°C and 50 bar in presence of different mineral assemblages (Andersson and Holm 2000). Decomposition rates of aspartic acid, leucine and alanine were found to be lower in the experiments containing PPT (pyrite-pyrrhotite-magnetite) mineral assemblage than in those without the minerals. Kohara et al. (1997) studied the stability of amino acids (alanine, β-alanine α-aminobutyric acid, glutamic acid, glycine, leucine, serine and valine) in ammonium chloride-hydrochloride acid aqueous solution at 200–350°C. The results showed a high recovery of amino acids, especially at a high hydrogen fugacity (0.8 bar) and temperature (300°C). Oligomerization experiments under hydrothermal conditions produced diglycine, triglycine, and diketopiperazine as the main products (Alagrov et al. 2002). The oligomerization is efficient in the process of temperature lowering, when the reaction solution at 200°C was injected into a cooler chamber at 0°C (Imai et al. 1999; Yokoyama et al. 2003).
The abiotic formation of various organic nitrogen compounds is possible under hydrothermal conditions. Thus, hydrothermal pyrolysis experiments were performed to assess condensation (dehydration) reactions to amide, ester, and nitrile functionalities from lipid precursors (Rushdi and Simoneit 2004). The availability of amide bonds is an important condition for peptide formation. Alkyl amides and nitriles have been obtained at high temperatures in the presence of excess aqueous ammonium species. Simple organic compounds containing nitrogen, such as amino acids, purines and pyrimidines, could be synthesized through the reaction pathways where hydrogen cyanide is central (Holm and Neubeck 2009). Reduced carbon and nitrogen precursor compounds for the synthesis of HCN may be formed under off-axis hydrothermal conditions in oceanic lithosphere in the presence of native Fe and Ni. After that HCN is available for further organic reactions, for instance, carbohydrates, nucleosides or even nucleotides, under alkaline conditions in hydrated mantle rocks. In natural settings cyanides were found in high temperature springs and gas jets in Kamchatka peninsula and Kuril islands (Mukhin et al. 1974).
The presence of mineral surfaces in hydrothermal systems can also facilitate synthesis and accumulation of organic matter. It has been shown that the synthesis of biologically important compounds, relevant for the origin of life, could be catalyzed by iron and nickel sulfides (Wachtershauser 1988; Russell 2003; Martin and Russell 2007). The formation of amino acids and nucleosides is possible under certain geological conditions through the reduction of CO2 and CO released from deep-sea vents. The formation of peptide bonds has also been demonstrated (Huber and Wächtershäuser 1998). The purine coding elements of RNA can be synthesized in the same abiotic reactions that produce amino acids (Levy et al. 2000). The equilibrium isotherm data on nucleic acids bases, adsorbed on surfaces of some sulfides and silicates at different temperatures shows that phase equilibria for purines, in particular, are far displaced toward adsorption on the solid phase, i.e. it would normally be useless to search for them in a fluid phase (Sowerby et al. 2002; Holm and Andersson 2005). Our experiment on the solution of several amino acids (glycine, L-alanine, L-aspartic acid, L-valine), nucleobases (adenine, cytosine, guanine and uracil), sodium phosphate, glycerol and myristic acid in a natural boiling water pool (T = 97°C, pH 3.1) in Kamchatka has led to the following result: with the exception of fatty acid, the compounds were adsorbed to the suspended clay particles (smectite, kaolinite, zeolite) and deposited in the several centimeter thick clay layer lining the pool (Deamer et al. 2006). The control experiments under pH 3.1 in the laboratory resulted in similar results. However, the nucleobases, amino acids and phosphate released from the clay into water under the alkaline conditions after adding NaOH.
The conclusion
Under certain conditions hydrocarbons (including aromatics), lipid compounds and simple amino acids can be synthesized and remain relatively stable in high temperature aqueous medium. They can exist in both soluble and insoluble forms in a fluid under the temperature 200–300°C and below. It has been experimentally demonstrate that these insoluble organics can form three-dimensional microsystems. Microdroplet emulsion (composed mainly of hydrocarbons) appears as a result of phase separating of oil from water at the temperature reduction in the fluid to about 200–300°C (Simoneit 2003). The temperature range of 150–200°C is suitable for the amino acid self-assembly into proteinoid microspheres, and lipids—into liposomes, if concentrations of these molecules reach some critical value (Fox and Dose 1975; Deamer et al. 2002, 2006; Simoneit 2004).
The synthesis of ribose and other sugars, nucleic acids and ATP needs lower temperatures and specific conditions. The thermal instability of ribose, which is a component of ATP and other nucleotides, is high: the half-lives for the decomposition at pH 7 and temperature 100°C is 73 min only (Larralde et al. 1995; Ehrenfreund et al. 2006). It is extremely difficult to conduct abiotic dehydration reactions in aqueous solutions to produce condensed phosphates, because of high water activity. The upper temperature limit for the experimental synthesis of nucleic acids is usually 50–60°C, and in the rare cases up to 90°C. Thus, heating of adenine and pyruvic aldehide in concentrated aqueous solution at 60°C led to a complete disappearance of adenine in few hours (Vergne et al. 2000).
Laboratory experiments on prebiotic chemistry are extremely important for better understanding of the origin-of-life process. However, in order to advance in this field we should take into consideration at least two distinctions between natural conditions and their laboratory simulations. First, a high temperature limit for the synthesis of nucleotide chains (50–60°C) refers to the processes in Vitro, i.e. to a chemical system without even primary properties of life. The same synthesis in Vivo, i.e. in a living biochemical system, sometimes proceeds at much higher temperatures. Thus, some species of Archaea can grow at the temperature 105–110°C, and even higher (Stetter 1995). Second, experienced researchers have concluded that auto replication of RNA is highly unlikely, unless it is sustained by the experimenter (Joyce et al. 1987; Horgan 1991). Below the author substantiates the thesis that a high temperature synthesis of nucleotides could be governed by the negentropy motive power, a “natural experimenter”, which arises in a microsystem at the inversion moment.
Prebiotic Chemistry in Hydrothermal Systems Relevant to the Inversion Approach
Consequences of the Inversion Approach for Prebiotic Chemistry and Early Biochemistry
There are some consequences of the inversion approach which are of importance for the chemical aspect of the origin-of-life scenario:
-
1.
The inversion of Fc/Sc balance in a prebiotic microsystem means a change of free energy gradient, from negative to positive, with respect to the environment. The same refers to information. In fact, such a transition might occur only in a three-dimensional prebiotic system, where the surplus of free energy can be accumulated and preserved in its central part.
-
2.
A bistate (oscillating) prebiotic microsystem should be composed of diverse organic compounds, capable of maintaining its internal heterogeneous structure, concentration gradients, and continuous reorganization of molecules through prebiotic reactions.
-
3.
The pre-inversion bistate microsystem had to exist under oscillating thermodynamic and physico-chemical conditions in the medium. As it was shown in the previous section, various organic compounds and macromolecules are very sensitive to changes of temperature, pressure, pH and redox potential in the environment. Due to external oscillations, a number of molecules experience the successive tendencies of synthesis and disintegration, providing a specific state of “stabilized instability” in the microsystem. These tendencies underlie metabolic processes in living organisms.
-
4.
A bistate prebiotic microsystem and its surroundings represent an inseparable system. Their integrity is sustained by substance, energy and (structural) information flows. At the inversion moment the microsystem becomes an active constituent of the whole system, and it is able to selectively extract organics from the environment. Thus, essential molecules and atoms might arrive in probionts from both internal and external sources.
-
5.
Right after the inversion moment, the negentropy motive power predominates in the microsystem and organizes internal reactions. The non-spontaneously directed synthesis of nucleic acids and ATP in emerging probionts might be much more efficient than the spontaneous synthesis of these macromolecules in a chemical system. In fact, the initial negentropy power might play the role of “a natural experimenter” promoting this kind of synthesis.
-
6.
It is a community of probionts (not a single probiont), that is considered by the author as a minimal self-sufficient unit of life. A number of three-dimensional prebiotic microsystems had to form a cluster in the liquid medium for life to appear.
In accordance with the inversion approach, it is reasonable to subdivide prebiotic chemical reactions into two categories:
-
A.
The prebiotic chemistry, which considers synthesis, accumulation and disintegration of organic matter in the origin of life context, including the formation of various prebiotic microsystems.
-
B.
The inversion chemistry, which links the prebiotic chemistry and the early biochemistry, investigating the prebiotic microsystems chemical transformation (inversion) into simplest living units.
Emergence and Development of Initial Prebiotic Microsystems in Hydrothermal Systems
It follows from “Organic Matter in Natural and Simulated Hydrothermal Medium” that hydrocarbons (including aromatics), lipids, and simple amino acids, as well as their precursors and biologically less important molecules, should be considered as relevant to the hydrothermal medium compounds, appropriate for the formation of initial prebiotic microsystems. All these classes of organic compounds can form three-dimensional microsystems through self-assembly. However, three-dimensional oil droplets of hydrocarbons do not contain biologically important molecules that could be transformed into primary forms of life. Low concentrations of amino acids revealed in natural hydrothermal settings hamper a self-assembly of proteinoide microspheres, which had been proposed by S. Fox as possible prebiotic models. Nevertheless, the presence of amino acid monomers and oligomers in prebiotic microsystems could be important for several reasons. These molecules represent a necessary initial component to start the nucleoprotein interaction. Besides, they might facilitate the accumulation of free energy in microsystems and preservation of structural (non-biological) information from the outside world. The important role of lipid amphiphilic bilayers that might form membrane-bounded compartments has been considered in prebiotic chemistry since the pioneering works by Deamer (1985, Deamer et al. 1994). Although there is no apparent evidence that abiotic lipid vesicles existed in natural hydrothermal solutions, the encapsulation of self-assembling lipid structures, like microtubules, is quite possible within three-dimensional prebiotic microsystems. Amphiphilic molecules might serve as boundary structures between the compartments inside an oscillating prebiotic microsystem, in this way they maintain its internal energetic gradients. By the inversion moment the microsystem had to obtain the liposome-like protective membrane that could prevent dissipation of the accumulated free energy surplus, and facilitate a selective extraction of substance from the environment. Combination of lipids and hydrocarbons could be conducive to this property rise. According to Deamer (2004), contemporary lipids typically contain hydrocarbon chains of 16–18 carbons in length. These chains provide the interior oily portion of lipid bilayer, which is almost an impermeable barrier for free diffusion of ions, such as sodium, potassium and protons. Summarizing the aforesaid, the three-dimensional aggregates, mainly composed of hydrocarbons, lipids and simple amino acids (or their precursors), should be considered as most appropriate prebiotic microsystems.
It follows from the experimental data, such initial organic microsystems might form within the approximate range of temperatures from 300 to 100°C. In hydrothermal fluids, highly concentrated brine, as well as organic substances, can segregate from the water phase under certain thermodynamic and physico-chemical conditions (Sharapov and Averkin 1990; Letnikov 1992). Thus, the phase separation of oil from water is a consequence of the fluid temperature reduction to 200–300°C. In the temperature window from ambient to ~300°C, hydrothermal oil parts for the bulk phase, micro droplet emulsion and true solution (Simoneit 2003). The organic matter rise of concentration in upper parts of hydrothermal reservoirs (due to gravitational partition) facilitates isolating of organic aggregates in the water medium.
The phase separation in a liquid system (segregation) begins with the appearance of numerous new phase nuclei in the matrix, with a simultaneous change of the matrix composition. The dynamics of this process was explored, in particular, on the example of melts by Delitsin et al. (1974). Nuclei of the new phase come to the state of turbulent move. They fuse and split up again, having a general tendency of the microsegregation transition into macrosegregation, with a common boundary between segregated phases. Exploration of oil deposits shows us a lot of geological structures, where the reservoirs of oil bulk phase and water phase are partitioned in space. However, the incomplete transition of microsegregation into macrosegregation might result in preservation of organic microscopic aggregates in the water solution. Agglomerations of various organic microsystems disseminated in water, from oil microdroplets to possible liposome-like structures, usually exist in convective conditions of hydrothermal systems. Convection of the fluid moving along open cracks and pores is a wide-spread phenomenon (Letnikov 1992; Baaske et al. 2007). Convection is more intensive at a high geothermal gradient, up to 23°C in the 30 m subsurface layer, in terrestrial hydrothermal fields (White 1957). The fluid agitation, due to the convection, may facilitate the emergence of chemical interactions between organic aggregates, within their associations.
A decrease in temperature, within the interval of 300–100°C, may result in diverse trends of the initial prebiotic microsystems evolution, dependent on the fluid composition, pressure, character of fluctuations, host rocks, etc. It is assumed, that the most favorable trend characterized by increase in the ratio of lipids and amino acids (or their precursors), and simple hydrocarbons, is the rise in diversity of chemical components and complication of the microsystems chemical reactions structure and network. Fluctuating conditions in hydrothermal systems could advance this kind of evolution. Thus, regular oscillations of redox potential in fluid may lead to regular changes of the tendencies to synthesis and decomposition of organic molecules. In addition, the temperature of the fluid approaching the surface is getting lower; correspondingly, a balance between synthesis and disintegration of (macro) molecules gradually changes.
According to the above mentioned experimental data, minor quantities of other biologically important molecules—sugars, nucleotides and ATP, or their precursors, might appear within and outside the prebiotic organic aggregates at the temperature below 100°C. Actually, a hydrothermal system includes liquid, gas-steam and solid (host rocks) phases. In “Organic Matter in Natural and Simulated Hydrothermal Medium” it is shown that nucleotides may accumulate on mineral surfaces, in hot acidic conditions. The condensed phosphates are present in volcanic gas-steam outcrops. A change of conditions in hydrothermal systems disturbs the phases balance, and it can initiate the organic substance redistribution between the phases. So, under changeable conditions in the hydrothermal medium a great number of re-combinations of organic molecules are possible both inside and outside prebiotic aggregates.
Formation of Living Probionts and Their Communities
Optimal Characteristics of Prebiotic Microsystems at the Fore-inversion Moment
Many kinds of organic molecules and aggregates could exist in hydrothermal medium at 300–100°C and below—up to70°C (a possible lowest temperature of the Hadean Ocean). The admissible list of these organics includes the main types of prebiotic models—liposomes, molecules of RNA World and aromatic World, organic molecules on the surface of minerals and in pores. However, the inversion approach makes certain demands of the types of prebiotic microsystems able to transform into the simplest living units: they should satisfy the six consequences listed in “Consequences of the Inversion Approach for Prebiotic Chemistry and Early Biochemistry”. From this point of view, one-dimensional chains of RNA, if they are in the aqueous medium (not inside a three-dimensional microsystem), have no chance to be inversed into probionts. According to the author’s opinion, the experiments on synthesis of nucleotide chains and their precursors are of great importance in the other respect. These experimenters have revealed potential chemical pathways to syntheses of nucleic acids, and they might take place, affected by the non-spontaneously directed negentropy motive power within prebiotic bistate microsystems. The two-dimensional chemolithotrophic metabolic systems on mineral surfaces do not seem an appropriate type of prebiotic models to inverse into primary living units. The organic substance accumulated in pores of sulfide minerals could be of use at the inversion stage of the origin-of-life process, if it gets into the fluid (for instance, due to changed conditions in the maternal medium). It is only in the fluid that prebiotic bistate microsystems can interact and assemble into the initial communities of probionts. The mineral-based origin-of-life scenarios provide a lot of data for considering the process of organic molecules re-distribution between the aqueous and solid phases.
The association of interacted three-dimensional organic microsystems, oscillating around the bifurcation point seems to be the most suitable ‘candidate’ for transformation into a primary minimal living system. The microsystems could be composed of hydrocarbons, lipids and amino acids (or/with precursors), in combination with less biologically important molecules. At the temperature about 100°C and below, minor quantities of sugars, nucleotides and ATP (or precursors) could be represented in prebiotic microsystems. In addition to C and H, the atoms of O, N, P, and S are to be available in the surroundings. The microsystems internal structure was bifurcated due to a lot of energy gradients. The microsystem oscillations around the bifurcation point provided a continuance of chemical reactions and its exchange by energy, substance and structural information with the outside world. It should be expected that cyclic and autocatalytic reactions, as well as the synthesis/decomposition of molecules, were wide-spread events in the microsystems because of the oscillating character of their existence.
Unlike in non-living systems, energy in living systems is temporarily preserved (as energy of chemical links and/or ionic gradients) for the period from its influx to release (Ho 1995). This general succession of energy transference in a living organism “generation (influx) → accumulation (storage) → release (utilization)” corresponds to the above suggested supposition that the living unit formation began with influx, generation and accumulation of free energy in a bistate prebiotic microsystem, and continued with fast release and utilization of the accumulated energy at the moment of inversion. The bifurcated heterogeneous structure of a bistate prebiotic microsystem facilitated reservation of free energy in various kinds of energy gradients (including gradients of substance concentrations, gradients of electric potentials, osmotic gradients). Another method of free energy storage is accumulation of high energy compounds, possibly including condensed phosphates available in a gas-steam phase of hydrothermal systems. The reversible cyclic and autocatalytic reactions are also appropriate for preserving a surplus of free energy.
According to the reconstruction of energy transferal in metabolic networks, two types of reactions are important for the basis of life (Hengeveld and Fedonkin 2007; Fedonkin 2008):
-
1)
Initial redox reactions connected with the transferal of electrons from donor to acceptor;
-
2)
Acidic-alkaline reactions of phosphates connected with transferal of protons that overbuilt on the initial ones.
Redox reactions in bistate prebiotic microsystems should be considered as the most substantial at the pre-inversion stage. Hydrogenation and dehydrogenation of molecules is determined by the electron exchange in the course of redox reactions. This implies an important role of hydrogen, its reactions catalyzed by metals like W, Fe, Ni. All these elements are characteristic of the hydrothermal medium. In contemporary forms of life the above mentioned reactions are characteristic of many enzyme co-factors (Fedonkin 2008). It is supposed that the initial gradients H+ appeared in the microsystems in the course of redox reactions at that stage.
Formation of Initial Living Units (Probionts)
The bistate prebiotic microsystem, got over the entropy barrier, acquires a new quality converting it into a living probiont. Being inside the thermodynamic niche of the initial life (Fig. 4, Ia), a probiont actively counteracts its return into the previous high-entropy state, promoting a development of the four key initial biological properties. It escapes the reversion, due to a continuous reorganization of its structure, the (bio) chemical reactions network, and the character of interaction with the environment. Its new organization works as the entropy pump sustaining a surplus of free energy and information (with respect to the environment) and efficiently exporting entropy. On the one hand, the system is open, as it exchanges by energy, substance and information with the environment. But, on the other hand, it is closed, being protected from the spontaneous dissipation of free energy and information into the outside world.
In a living cell a signal (energy impulse) is intensified by cascades of biochemical reactions, in which one enzyme, being a product of the previous catalytic reaction, catalyzes the formation of the next enzyme, catalyzing, in its turn, the synthesis of the following one, and so on (Fedonkin 2008). The formation of catalytic cascades under nonequilibrium conditions during the origin-of-life process was considered by Feistel and Ebeling (2011). According to the inversion approach, the primary cascade of biochemical reactions emerged in the process of avalanche-like release of free energy stored in prebiotic bistate microsystems surmounting the entropy barrier. The autocatalytic reactions reorganized into ascending cascades producing more and more free energy. Under such a transformation local low entropy structures expanded and organized into the primary biological unit (Fig. 7). The surplus free energy began its circulation within the biochemical reactions network, and in this way maintained a positive free energy gradient of a probiont with respect to the environment (the same applies to information).
Inversion of the balance between the contributions of spontaneous and non-spontaneous processes in a chemical system. Left—a system with prevalence of spontaneous processes, right—one with prevalence of non-spontaneous processes. ‘a’—areas with prevalence of non-spontaneous processes containing low entropy structures that produce free energy and information; ‘b’—areas with prevalence of spontaneous processes containing high entropy structures; ‘c’ associative links between high entropy structures and low entropy structures. F—free energy, F+—input of free energy, F−—loss (dissipation) of free energy
After the original negentropy impulse had been exhausted, initial probionts had to display activity with respect to the environment, not to descend beneath the entropy barrier. In other words, they had to make the entropy pump work. Occupying an active position in the outside world, probionts reorganized the substance exchange in the way that would allow them to selectively extract a higher energy matter, excreting a lower energy substance. Simultaneously, the structure and functions of the lipid bilayers capsule were reorganized in the way they could selectively extract high-energy molecules and protect a probiont from a spontaneous loss of energy. Amphiphilic bilayer molecules also participated in the construction of internal compartments that stabilized the internal ionic gradients and enlarged the energetic capacity of a probiont.
Circulative autocatalytic reactions in low-entropy structures had led to the proton pump formation developed from initial proton gradients. The incessant transfer of protons gave rise to more efficient acidic-alkaline reactions of phosphates (up to ATP formation) built on the redox type of reactions. In contemporary cells, H+ gradients are used in the process of ATP generation; various microbial enzymes perform H+ transfer (Fedonkin 2008). The acidic-alkaline reactions launched the condensation-hydrolysis cycle. Hydrolysis of ATP (to ADP and AMP) proceeded with a release of energy and adsorption of water. The reactions of biopolymers condensation (peptides, polysaccharides, polynucleotides), on the contrary, proceed with a release of water and utilization of energy. These reactions are thermodynamically and chemically coupled, allowing for the primary living units to minimize the internal entropy production (Galimov 2006). The reduction of ATP energy took place through phosphorylation of AMP and ADP: ADP + Pi (+ E) → ATP. The energy source for ATP reducing could vary, from geochemical energy to light quanta (Galimov 2006). The reactions of phosphates provided the energetic basis for oligomerization and polymerization of amino acids and nucleic acids. The integrative organization of a probiont prescribed any catalyst to catalyze a certain reaction. Such a method of organization could appear only due to the emerged bio-informational process.
The probiont dissymmetrical heterogeneous structure facilitated the formation of stereo specificity—combination of L-amino acids and D-sugars. In case of spontaneous D- and L-sugars alternation in nucleic acids, the complementary pairs could not be formed. In case of amino acid racemization , they could not recognize each other within the polypeptide spiral, which was considered, in particular, by Galimov (2006). Probably, at the origin of life the opposite chirality might have emerged as well.
Finally, a reconstruction of the oscillating prebiotic microsystem transition into the primary living unit is represented in Fig. 1 bottom, Fig. 8a–d. The pictures show the inversion of balance “contribution of free energy / contribution of entropy” inversion; the same illustrations could be made for the inversion of balance “contribution of information / contribution of informational entropy”. The oscillating bistate microsystem is characterized by an exchange of energy and matter with the outside world; a tendency to dichotomy; continuous reactions resulted in free energy accumulation and preservation (Fig. 8a). Changes in the outside world stress the microsystem, provoking a release of preserved free energy. As a result, the total of internal and external energy contributions prevails over dissipation (Fig. 8b). The resulting direction of free energy flow reverses from the external to internal one (Fig. 8c). In this way, the microsystem undergoes thermodynamic inversion, importing free energy and exporting entropy (Fig. 8d). The appearance of bioinformation in the context of the inversion approach is considered in another publication by the author (Kompanichenko 2012b).
Reconstruction of a prebiotic microsystem transition into living unit. Fint (internal) and Fext (external) free energy contributions into a prebiotic system; ∆ F+ (positive) and ∆ F− (negative) free energy gradients of the system with respect to the surroundings; arrows—directions of free energy and entropy flows
Primary Communities of Probionts
As it follows from the inversion approach, it was a community of probionts (not a single probiont) that represented a minimal self-sufficient unit of life. The two arguments in support of this notion are as follows:
-
1.
The preceding life bifurcation process proceeded according to the statistical physical mechanism. Through bifurcation any chemical system has a chance (i.e. a certain probability) to develop, in accordance with the A, B, B’, C’, C” trends (Fig. 2). But the evolution of a system to the living state is possible only under the condition of its continuous stay near the bifurcation point. Due to the same laws, only a part of the bistate prebiotic microsystems might overcome the entropy barrier, the rest of them excluded from the origin-of-life process. So, a great number of the initial prebiotic microsystems had to be involved in the origin-of-life process at its earliest stage, to produce only a small number of probionts.
-
2.
Segregation of three-dimensional organic (prebiotic) microsystems in aqueous medium could occur under high concentration of the organic matter in it. The segregation process resulted in the emergence of numerous organic microsystems that, under the same conditions, had to produce a considerable number of interacting probionts.
On the lifeless Earth prebiotic organic microsystems were involved into natural geochemical cycles. The inversion of microsystems in local hydrothermal zones initiated the process of building the probionts metabolic networks into thermodynamic and physico-chemical gradients of the medium. Being an active component with respect to the abiotic environment, the probionts transformed the geochemical cycles into biogeochemical ones. The hydrothermal medium is usually anisotropic, and it is characterized by variable gradients of temperature, pressure, electric potentials, concentrations of chemical components, etc. The evolution of probionts in such heterogeneous conditions led to the formation of heterogeneous communities of various probionts and their groups occupying different ecological niches. The interaction between different complementary groups of probionts resulted in the emergence of the community functioning as a stable self-sufficient unit of life. According to the conception by Zavarzin, biogeochemical cycles of the biosphere might be launched only by different obverse groups of microorganisms (Zavarzin 2006). The contemporary prokaryotic communities catalyze biogeochemical cycles, all the reactions conjugated into a common system.
A theoretical reconstruction of the evolution of probionts to progenotes, and later to contemporary prokaryotes was made in another work by the author (Kompanichenko 2009b).
Conclusion
The inversion approach offers a new way for laboratory experiments on the origin of life. The theoretically substantiated origin-of-life process (including both prebiotic and biotic stages) should be tested in an experimental system with fluctuating conditions. Modeling the prebiotic stage, the behavior of relevant organic compounds (hydrocarbons, lipids, amino acids, nucleic acids) should be explored in the fluctuating medium simulating the fluid rising in the thermo gradient field of the Earth’s upper crust. The continuous synthesis of organic (marco) molecules and self-assembly of organic microsystems (i.e. a change in the synthesis/oligomerization and destruction tendencies, due to external fluctuations) are the most interesting processes to investigate. Thus, the synthesis and oligomerization of glycine takes place in simulated hydrothermal conditions, but synthesized (macro) molecules have a tendency to destruction (Aubrey et al. 2009; Cleaves et al. 2009). However, as it follows from the inversion approach, these molecules may complicate and reorganize under optimal fluctuations and changes of temperature. These experiments could serve as a good basis for the next experimental attempts to obtain probionts.
References
Aubrey AD, Cleaves HJ, Bada JL (2009) The role of submarine hydrothermal systems in the synthesis of amino acids. Orig Life Evol Biosph 39:91–108
Alagrov DK, Chigeru D, Tsujii K, Horikoshi K (2002) Oligomerization of glycine in supercritical water with special attention to the origin of life in deep-sea hydrothermal systems. Prog Biotechol 19:631–636
Andersson E, Holm NG (2000) The stability of some selected amino acids under attempted redox constrained hydrothermal conditions. Orig Life Evol Biosph 30:9–23
Baaske P, Weinert FM, Duhr S, Lemke KH, Russell MJ, Braun D (2007) Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc Natl Acad Sci USA 104(22):9346–9351
Baltscheffsky H (1997) Major «anastrophes» in the origin and early evolution of biological energy conversion. J Theor Biol 187:495–501
Basiuk VA, Navarro-Gonzalez R (1996) Possible role of volcanic ash-gas clouds in the Earth’s prebiotic chemistry. Orig Life Evol Biosph 26:173–194
Berndt ME, Allen DW, Seyfried WE Jr (1996) Reduction of CO2 during serpentinization of olivine at 300°C and 500 bar. Geology 24:351–354
Bernhardt G, Ludemann HD, Jaenicke R (1984) Biomolecules are unstable under “black smoker” conditions. Naturwissenschaften 71:583–586
Cleaves HJ, Aubrey AD, Bada JL (2009) An evaluation of critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig Life Evol Biosph 39:109–126
Corliss JB, Baross JA, Hoffman SE (1981) An hypothesis concerning the relationship between submarine hot springs and the origin of life on the Earth. Oceanol Acta SP 4:59–69
Deamer DW (1985) Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 317:792–794
Deamer DW (2004) Prebiotic amphiphilic compounds. In: Seckbach J (ed) Origins. Kluwer, Netherlands, pp 75–89
Deamer DW, Harang Mahon E, Bosco J. (1994) Self-assembly and function of primitive membrane structures. In: Bengtson S (ed) Early life on earth. Nobel Symposium 84, Columbia University Press, New York, pp. 107–123
Deamer D, Dworkin JP, Sandford SA, Bernstein MP, Allamandola LJ (2002) The first cell membranes. Astrobiology 2:371–382
Deamer D, Singaram S, Rajamani S, Kompanichenko V, Guggenheim S (2006) Self-assembly processes in the prebiotic environment. Philos Trans R Soc B 361(1474):1809–1818
De Duve C (2002) Life is what is common to all living beings. In: Palyi G, Zucci C, Caglioti L (eds) Fundamentals of life. Elsevier, Paris, pp 26–27
Delitsin LM, Melentyev BM, Delitsina LV (1974) Segregation in melts—insipience, development, and stabilization. Doklady Acad Sci USSR 219(1):190–193, In Russian
Didyk BM, Simoneit BRT (1990) Petroleum characteristics of the oil in a Guamaras basin hydrothermal chimney. In: Simoneit BRT (ed) Organic Matter Alteration in Hydrothermal Systems—Petroleum Generation, Migration and Biogeochemistry. Appl Geochem 5:29–40
Ebeling W, Engel A, Feistel R (1990) Physik der evolutionsprozesse. Akademie-Verlag, Berlin, In German
Ehrenfreund P, Rasmussen S, Cleaves J, Chen L (2006) Experimentally tracing the key steps in the origin of life: the aromatic world. Astrobiology 6(3):490–520
Elitzur A (2002) Life is what is common to all living beings. In: Palyi G, Zucci C, Caglioti L (eds) Fundamentals of life. Elsevier, Paris, pp 27–28
Fedonkin MA (2008) Ancient biosphere: the origin, trends and events. Russ Jour Earth Sci 10:1–9. doi:10.2205/2007ES000252
Feistel R., Ebeling W. (2011) Physics of Self-organization and Evolution. Wiley, VCH
Fox S, Dose K (1975) Molecular evolution and the origin of life. Dekker, New York
Galimov EM (2006) Phenomenon of life: between equilibrium and nonlinearity. Origin and Principles of Evolution. Editorial URSS, Moscow (In Russian)
Gladyshev GP (1995) About dynamic direction of biological evolution. Izvestia Russ Acad Sci, Ser Biol 1:5–14
Haken H (1978) Synergetics. Springer, Berlin
Haken H (2003) Special issue: nonlinear phenomena in complex systems 5(4)
Hengeveld R, Fedonkin MA (2007) Bootstrapping the energy flow in the beginning of life. Acta Biotheor 55:181–226
Hennet RJ-C, Holm NG, Engel MH (1992) Abiotic synthesis of amino acids under hydrothermal conditions and the origin of life: a perpetual phenomenon? Naturwissenschaften 79:361–365
Ho M-W (ed) (1995) Living processes. Book 2: Bioenergetics. Open University Press, Milton Keynes
Holm NG, Andersson E (2005) Hydrothermal simulation experiments as a tool for studies for the origin of life on Earth and other terrestrial planets: a review. Astrobiology 5(4):444–460
Holm NG, Charlou JL (2001) Initial indications of abiotic formation of hydrocarbons in the Rainbow ultramafic hydrothermal system, Mid-Atlantic Ridge. Earth Planet Sci Lett 191:1–8
Holm NG, Neubeck A (2009) Reduction of nitrogen compounds in oceanic basement and its implications for HCN formation and abiotic organic synthesis. Geochem Trans 10:9. doi:10.1186/1467-4866-10-9
Horgan J (1991) Near cradle of life. V Mire Nauki 4:68–79 in Russian (Translated from: 1991). Sci Am 264(2):68–79
Huber C, Wächtershäuser G (1998) Peptides by activation of amino acids with CO on (Ni, Fe)S surfaces: implications for the origin of life. Science 281:670–672
Imai E, Honda H, Hatori K, Brack A, Matsuno K (1999) Elongation of oligopeptides in a simulated hydrothermal system. Science 283:831–833
Isidorov VA, Zenkevich IG, Karpov GA (1992) Volatile organic compounds in steam-gas outflows of several volcanoes and hydrothermal systems in Kamchatka. Volc Seis 13(3):287–293
Joyce GF, Schwartz AW, Miller SL, Orgel LE (1987) The case for an ancestral genetic system involving simple analogs of nucleotides. Proc Natl Acad Sci USA 84:4398–4402
Kelley DS (1996) Methane-rich fluids in the oceanic crust. J Geophys Res 101:2943–2962
Knauth LP, Lowe DR (2003) High Archaean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. Geol Soc Am Bull 115(5):566–580
Kohara M, Gamo T, Yanagawa H, Kobayashi K (1997) Stability of amino acids in simulated hydrothermal vent environments. Chem Lett 1997:1053–1054
Kompanichenko VN (2003) Distinctive properties of biological systems: the all-round comparison with other natural systems. Front Perspect 12(1):23–35
Kompanichenko VN (2004) Systemic approach to the origin of life. Front Perspect 13(1):22–40
Kompanichenko VN (2008) Three stages of the origin-of-life process: bifurcation, stabilization and inversion. Int J Astrobiol 7(1):27–46
Kompanichenko VN (2009a) Changeable hydrothermal media as a potential cradle of life on a planet. Planet Space Sci 57:468–476
Kompanichenko VN (2009b) Way from physical nonequilibrium to biological evolution. Chem Phys Res J 2(3):199–214
Kompanichenko VN (2009c) Organic matter in hydrothermal systems of Kamchatka: relevance to the origin of life. Orig Life Evol Biosph 39:338–339
Kompanichenko VN (2012a) Thermodynamic inversion and self-reproduction with variations: integrated view on the life-nonlife border. J Biomol Struct Dyn 29(4):637–639
Kompanichenko VN (2012b) Origin of life by thermodynamic inversion: a universal process. In: Seckbach J (ed) Genesis—In the beginning: precursors of life, chemical models and early biological evolution. Springer, Dordrecht
Kralj P, Kralj P (2000) Thermal and mineral waters in north-eastern Slovenia. Environ Geol 39(5):488–498
Larralde R, Robertson M, Miller S (1995) Rates of decomposition of ribose and other sugars: implications for chemical evolution. Proc Natl Acad Sci USA 92:8158–8160
Letnikov FA (1992) Synergetics of geological systems. Nauka, Novosibirsk (In Russian)
Levy M, Miller SL, Brinton K, Bada JL (2000) Prebiotic synthesis of adenine and amino acids under Europa-like conditions. Icarus 145:609–613
Lin S-K (1996) Correlation of entropy with similarity and symmetry. J Chem Inf Comp Sci 36:367–376
Markhinin EK, Podkletnov NE (1977) The phenomenon of formation of prebiological compounds in volcanic processes. Orig Life 3:225–235
Marshall WL (1994) Hydrothermal synthesis of amino acids. Geochim Cosmochim Acta 58:2099–2106
Martin W, Russell JM (2007) On the origin of biochemistry at an alkaline hydrothermal vent. Philos Trans R Soc B 362:1887–1925
McCollom TM, Ritter G, Simoneit BRT (1999) Lipid synthesis under hydrothermal conditions by Fischer-Tropsch type reactions. Orig Life Evol Biosph 29:152–166
McCollom TM, Seewald JS (2001) A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim Cosmochim Acta 65:3769–3778
Miller SL, Bada JL (1988) Submarine hot springs and the origin of life. Nature 334:609–611
Morrison D (2001) The NASA astrobiology program. Astrobiology 1(1):3–13
Mukhin LM, Vasiljev VN, Ponomarev VV (1974) Discovery of hydrogen cyanide and its derivatives in regions of active volcanism. Doklady Acad Sci USSR 215(5):1253–1254
Mukhin LM, Bondarev VB, Vakin EA, Iljukhina II, Kalinichenko VI, Milekhina EI, Safonova EN (1979) Amino acids in hydrothermal systems in Southern Kamchatka. Doklady Acad Sci USSR 244(4):974–977
Nicolis G, Prigogine I (1977) Self-organization in Nonequilibrium Systems. Wiley, New York
Pace NR (1991) Origin of life—facing up to the physical setting. Cell 65:531–533
Palyi G, Zucci C, Caglioti L (eds) (2002) Fundamentals of life. Elsevier, Paris
Polishchuk R (2002) Life as a negentropy current and infinity problem. In: Palyi G, Zucci C, Caglioti L (eds) Fundamentals of life. Elsevier, Paris, pp 141–152
Prigogine I, Stengers I (1984) Order out of chaos. Bantam, New York
Robert F, Chaussidon M (2006) A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 443:969–972
Rushdi AI, Simoneit BRT (2001) Lipid formation by aqueous Fischer-Tropsch type synthesis over a temperature range of 100–400°C. Orig Life Evol Biosph 31:103–118
Rushdi AI, Simoneit BRT (2004) Condensation reactions and formation of amides, esters, and nitriles under hydrothermal conditions. Astrobiology 4(2):211–224
Russell MJ (2003) The importance of being alkaline. Science 302:580–581
Russell MJ, Hall AJ, Boyce AJ, Fallick AE (2005) On hydrothermal convection and the emergence of life. Econ Geol 100:419–438
Schwartzman DW, Lineweaver CH (2004) The hyperthermophilic origin of life revisited. Biochem Soc Trans 32(2):168–171
Selye H (1974) Stress without distress. JB Lippincott Company, Philadelphia
Sharapov VN, Averkin JuA (1990) Dynamics of heat- and mass-exchange in orto-magmatic fluid systems. Nauka, Novosibirsk (In Russian)
Shields GA, Kasting JF (2007) Evidence for hot early ocean? Nature 447. doi:10.1038/nature05831
Shock EL, McCollom TM, Schulte MD (1998) The emergence of metabolism from within hydrothermal systems. In: Wiegel J, Adams MWW (eds) Thermophiles: the keys to molecular evolution and the origin of life. Taylor and Francis, Washington, pp 59–76
Simoneit BRT (1993) Aqueous high-temperature and high-pressure organic geochemistry of hydrothermal vent systems. Geochim Cosmochim Acta 57:3231–3243
Simoneit BRT (2003) Petroleum generation, extraction and migration and abiogenic synthesis in hydrothermal systems. In: Ikan R (ed) Natural and laboratory simulated thermal geochemical processes. Kluwer, Netherlands, pp 1–30
Simoneit BRT (2004) Prebiotic organic synthesis under hydrothermal conditions: an overview. Adv Space Res 33:88–94
Simoneit BRT, Lein AY, Peresupkin VI, Osipov GA (2004) Composition and origin of hydrothermal petroleum and associated lipids in the sulfide deposits of the Rainbow field (Mid-Atlantic Ridge at 36ºN). Geochim Cosmochim Acta 68(10):2275–2294
Sowerby SJ, Petersen JB, Holm NG (2002) Primordial coding of amino acids by adsorbed purine bases. Orig Life Evol Biosph 32:35–46
Stetter KO (1995) Microbial life in hyperhermal environments. ASM News 61(6):328–340
Strazewski P (2007) How did translation occur? Orig Life Evol Biosph 37:399–401
Vergne J, Dumas L, Decout J-L, Maurel M-C (2000) Possible prebiotic catalysts formed from adenine and aldehyde. Planet Space Sci 48:1139–1142
Vernadsky VI (1980) Problems of biogeochemistry. Nauka, Moscow (In Russian)
Wächtershäuser G (1988) Before enzymes and templates: theory of surface metabolism. Microbiol Rev 52:452–484
Washington J (2000) The possible role of volcanic aquifers in prebiotic genesis of organic compounds and RNA. Orig Life Evol Biosph 30:53–79
White DE (1957) Thermal waters of volcanic origin. Bull Geol Soc Am 63
Yamagata Y, Watanabe H, Saitoh M, Namba T (1991) Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 352:516–519
Yokoyama S, Koyama A, Nemoto A, Honda H, Hatori K, Matsuno K (2003) Amplification of diverse catalytic properties of evolving molecules in a simulated hydrothermal environment. Orig Life Evol Biosph 33:589–595
Yuen G, Kvenvolden K (1973) Monocarboxylic acids in Murray and Murchison carbonaceous meteorites. Nature 246:301–303
Zavarzin GA (2006) Does evolution make the essence of biology? Herald Russ Acad Sci 76(3):292–302
Acknowledgments
The author is very grateful to Alla Voronina for her assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kompanichenko, V.N. Inversion Concept of the Origin of Life. Orig Life Evol Biosph 42, 153–178 (2012). https://doi.org/10.1007/s11084-012-9279-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-012-9279-0