Abstract
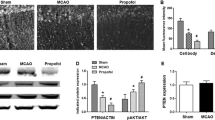
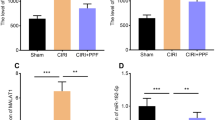
Propofol and ketamine may provide certain degree of neuroprotection, but the underlying mechanism remains unclear to date. The cAMP response element-binding protein (CREB) was proposed that its phosphorylation at Ser133 (P-CREB) constituted a convergence point involved in neuroprotection. The purpose of this study was to determine whether different dosages of propofol and ketamine could provide neuroprotection against permanent middle cerebral artery occlusion (MCAO)-induced ischemic injuries and the involvement of P-CREB. Eighty adult male BALB/c mice that underwent 6 h MCAO were randomly divided into eight groups: Sham-operation; MCAO + saline; MCAO + 25, 50, 100 mg/kg propofol; and MCAO + 25, 50, 100 mg/kg ketamine (intraperitoneal injection 30 min following MCAO). We found that 50, 100 (not 25) mg/kg propofol, and 25 (not 50 and 100) mg/kg ketamine could significantly reduce the infarct volume, edema ratio and neurological deficit (n = 10 per group) as well as inhibit the decrease of P-CREB level in peri-infarct region when compared with that of MCAO + saline group (n = 6 per group). In addition, the results of double-labeled immunofluorescent staining showed that P-CREB co-localized with neuron-specific marker, NeuN, in the peri-infarct region of 50 mg/kg propofol and 25 mg/kg ketamine treated 6 h MCAO mice (n = 4 per group). These results suggested that inhibition of neuron-specific P-CREB dephosphorylation in the peri-infarct region is involved in high dose propofol and low dose ketamine-induced neuroprotection of 6 h MCAO mice.





Similar content being viewed by others

References
Ludbrook GL, Visco E, Lam AM (2002) Propofol: relation between brain concentrations, electroencephalogram, middle cerebral artery blood flow velocity, and cerebral oxygen extraction during induction of anesthesia. Anesthesiology 97:1363–1370
Kaisti KK, Metsahonkala L, Teras M, Oikonen V, Aalto S, Jaaskelainen S, Hinkka S, Scheinin H (2002) Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology 96:1358–1370
Priebe HJ (2007) Aneurysmal subarachnoid haemorrhage and the anaesthetist. Br J Anaesth 99:102–118
Ishiyama T, Shibuya K, Ichikawa M, Masamune T, Kiuchi R, Sessler DI, Matsukawa T (2010) Cerebral pial vascular changes under propofol or sevoflurane anesthesia during global cerebral ischemia and reperfusion in rabbits. J Neurosurg Anesthesiol 22:207–213
Chen L, Xue Z, Jiang H (2008) Effect of propofol on pathologic time-course and apoptosis after cerebral ischemia-reperfusion injury. Acta Anaesthesiol Scand 52:413–419
Wang HY, Wang GL, Yu YH, Wang Y (2009) The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res 1297:177–184
Bayona NA, Gelb AW, Jiang Z, Wilson JX, Urquhart BL, Cechetto DF (2004) Propofol neuroprotection in cerebral ischemia and its effects on low-molecular-weight antioxidants and skilled motor tasks. Anesthesiology 100:1151–1159
Grasshoff C, Gillessen T (2002) The effect of propofol on increased superoxide concentration in cultured rat cerebrocortical neurons after stimulation of N-methyl-d-aspartate receptors. Anesth Analg 95:920–922
Wilson JX, Gelb AW (2002) Free radicals, antioxidants, and neurologic injury: possible relationship to cerebral protection by anesthetics. J Neurosurg Anesthesiol 14:66–79
Berns M, Seeberg L, Schmidt M, Kerner T (2009) High-dose propofol triggers short-term neuroprotection and long-term neurodegeneration in primary neuronal cultures from rat embryos. J Int Med Res 37:680–688
Buggy DJ, Nicol B, Rowbotham DJ, Lambert DG (2000) Effects of intravenous anesthetic agents on glutamate release: a role for GABAA receptor-mediated inhibition. Anesthesiology 92:1067–1073
Kobayashi M, Takeda Y, Taninishi H, Takata K, Aoe H, Morita K (2007) Quantitative evaluation of the neuroprotective effects of thiopental sodium, propofol, and halothane on brain ischemia in the gerbil: effects of the anesthetics on ischemic depolarization and extracellular glutamate concentration. J Neurosurg Anesthesiol 19:171–178
Adembri C, Venturi L, Tani A, Chiarugi A, Gramigni E, Cozzi A, Pancani T, De Gaudio RA, Pellegrini-Giampietro DE (2006) Neuroprotective effects of propofol in models of cerebral ischemia: inhibition of mitochondrial swelling as a possible mechanism. Anesthesiology 104:80–89
Grasshoff C, Gillessen T (2005) Effects of propofol on N-methyl-d-aspartate receptor-mediated calcium increase in cultured rat cerebrocortical neurons. Eur J Anaesthesiol 22:467–470
Takeshita H, Okuda Y, Sari A (1972) The effects of ketamine on cerebral circulation and metabolism in man. Anesthesiology 36:69–75
Xue QS, Yu BW, Wang ZJ, Chen HZ (2004) Effects of ketamine, midazolam, thiopental, and propofol on brain ischemia injury in rat cerebral cortical slices. Acta Pharmacol Sin 25:115–120
Zhang C, Shen W, Zhang G (2002) N-methyl-d-aspartate receptor and L-type voltage-gated Ca(2+) channel antagonists suppress the release of cytochrome c and the expression of procaspase-3 in rat hippocampus after global brain ischemia. Neurosci Lett 328:265–268
Basagan-Mogol E, Buyukuysal RL, Korfali G (2005) Effects of ketamine and thiopental on ischemia reoxygenation-induced LDH leakage and amino acid release from rat striatal slices. J Neurosurg Anesthesiol 17:20–26
Wang L, Jing W, Hang YN (2008) Glutamate-induced c-Jun expression in neuronal PC12 cells: the effects of ketamine and propofol. J Neurosurg Anesthesiol 20:124–130
Carlezon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28:436–445
Ferrer I, Friguls B, Dalfo E, Planas AM (2003) Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. Acta Neuropathol 105:425–437
Zhang QG, Wang RM, Han D, Yang LC, Li J, Brann DW (2009) Preconditioning neuroprotection in global cerebral ischemia involves NMDA receptor-mediated ERK-JNK3 crosstalk. Neurosci Res 63:205–212
Zhang ZH, Xi GM, Li WC, Ling HY, Qu P, Fang XB (2010) Cyclic-AMP response element binding protein and tau are involved in the neuroprotective mechanisms of nerve growth factor during focal cerebral ischemia/reperfusion in rats. J Clin Neurosci 17:353–356
Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW (2010) Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One 5:e9851
Zhang ZH, Fang XB, Xi GM, Li WC, Ling HY, Qu P (2010) Calcitonin gene-related peptide enhances CREB phosphorylation and attenuates tau protein phosphorylation in rat brain during focal cerebral ischemia/reperfusion. Biomed Pharmacother 64:430–436
Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP (2005) CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab 25:234–246
Chen A, Liao WP, Lu Q, Wong WS, Wong PT (2007) Upregulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats—a proteomics approach. Neurochem Int 50:1078–1086
Rodriguez R, Santiago-Mejia J, Gomez C, San-Juan ER (2005) A simplified procedure for the quantitative measurement of neurological deficits after forebrain ischemia in mice. J Neurosci Methods 147:22–28
Wexler EJ, Peters EE, Gonzales A, Gonzales ML, Slee AM, Kerr JS (2002) An objective procedure for ischemic area evaluation of the stroke intraluminal thread model in the mouse and rat. J Neurosci Methods 113:51–58
Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ (1998) Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke 29:1037–1046
Cui X, Li J, Li T, Ji F, Bu X, Zhang N, Zhang B (2009) Propofol and ketamine-induced anesthetic depth-dependent decrease of CaMKII phosphorylation levels in rat hippocampus and cortex. J Neurosurg Anesthesiol 21:145–154
Jia J, Wang X, Li H, Han S, Zu P, Li J (2007) Activations of nPKCepsilon and ERK1/2 were involved in oxygen-glucose deprivation-induced neuroprotection via NMDA receptors in hippocampal slices of mice. J Neurosurg Anesthesiol 19:18–24
Jiang J, Yang W, Huang P, Bu X, Zhang N, Li J (2009) Increased phosphorylation of Ets-like transcription factor-1 in neurons of hypoxic preconditioned mice. Neurochem Res 34:1443–1450
Wang J, Yang X, Camporesi CV, Yang Z, Bosco G, Chen C, Camporesi EM (2002) Propofol reduces infarct size and striatal dopamine accumulation following transient middle cerebral artery occlusion: a microdialysis study. Eur J Pharmacol 452:303–308
Zheng YY, Lan YP, Tang HF, Zhu SM (2008) Propofol pretreatment attenuates aquaporin-4 over-expression and alleviates cerebral edema after transient focal brain ischemia reperfusion in rats. Anesth Analg 107:2009–2016
Chang ML, Yang J, Kem S, Klaidman L, Sugawara T, Chan PH, Adams JD Jr (2002) Nicotinamide and ketamine reduce infarct volume and DNA fragmentation in rats after brain ischemia and reperfusion. Neurosci Lett 322:137–140
Zhan RZ, Qi S, Wu C, Fujihara H, Taga K, Shimoji K (2001) Intravenous anesthetics differentially reduce neurotransmission damage caused by oxygen-glucose deprivation in rat hippocampal slices in correlation with N-methyl-d-aspartate receptor inhibition. Crit Care Med 29:808–813
Church J, Zeman S, Lodge D (1988) The neuroprotective action of ketamine and MK-801 after transient cerebral ischemia in rats. Anesthesiology 69:702–709
Bourgoin A, Albanese J, Wereszczynski N, Charbit M, Vialet R, Martin C (2003) Safety of sedation with ketamine in severe head injury patients: comparison with sufentanil. Crit Care Med 31:711–717
Reicher D, Bhalla P, Rubinstein EH (1987) Cholinergic cerebral vasodilator effect of ketamine in rabbits. Stroke 18:445–449
Reeker W, Werner C, Mollenberg O, Mielke L, Kochs E (2000) High-dose S(+)-ketamine improves neurological outcome following incomplete cerebral ischemia in rats. Can J Anaesth 47:572–578
Himmelseher S, Durieux ME (2005) Revising a dogma: ketamine for patients with neurological injury? Anesth Analg 101:524–534
Bourgoin A, Albanese J, Leone M, Sampol-Manos E, Viviand X, Martin C (2005) Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain-injured patients. Crit Care Med 33:1109–1113
Kim DH, Kim S, Jung WY, Park SJ, Park DH, Kim JM, Cheong JH, Ryu JH (2009) The neuroprotective effects of the seeds of Cassia obtusifolia on transient cerebral global ischemia in mice. Food Chem Toxicol 47:1473–1479
Kitagawa K (2007) CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J 274:3210–3217
Sugiura S, Kitagawa K, Omura-Matsuoka E, Sasaki T, Tanaka S, Yagita Y, Matsushita K, Storm DR, Hori M (2004) CRE-mediated gene transcription in the peri-infarct area after focal cerebral ischemia in mice. J Neurosci Res 75:401–407
Acknowledgments
This work was supported by the following grants: National Natural Science Foundation of China (30871219, 31071048 and 81070976), China 973 Pre-program (2011CB512109), Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of Beijing Municipality (PHR200906116), and Ph.D. Programs Foundation of Ministry of Education of China (20091107110001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Luowa Shu and Tianzuo Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shu, L., Li, T., Han, S. et al. Inhibition of Neuron-Specific CREB Dephosphorylation is Involved in Propofol and Ketamine-Induced Neuroprotection Against Cerebral Ischemic Injuries of Mice. Neurochem Res 37, 49–58 (2012). https://doi.org/10.1007/s11064-011-0582-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0582-3