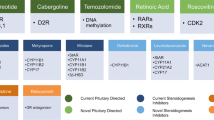
Abstract
Cushing’s disease (CD), caused by excess adrenocorticotropin secretion from tumorous pituitary corticotrophs, is associated with substantial morbidity and mortality. The primary, definitive therapy for patients with CD is selective pituitary adenomectomy, generally performed via a transsphenoidal approach. Medical therapy has an important adjunctive role in the management of patients with CD, including preoperative patient preparation in patients with severe disease, and temporizing management of hypercortisolism while awaiting the effects of radiation therapy to occur in patients who are not in remission postoperatively. Medical therapy can also be used in patients with hypercortisolism of unclear origin or in the few patients who decline or are unfit for surgery. Available medical options for patients with CD include centrally acting agents (cabergoline and pasireotide), steroidogenesis inhibitors (ketoconazole, metyrapone, mitotane and etomidate) and a glucocorticoid receptor antagonist (mifepristone). Pasireotide and mifepristone have been recently granted regulatory approval in some countries for use in patients with CD, whereas other medications are used “off label” in this patient population. As clinical trials using comparator agents have not been reported, the choice between different medications is based on patient characteristics and preference. Despite impressive advances in pharmacotherapy for patients with CD, much remains to be done. The long term efficacy and safety of medical therapies for hypercortisolism need to be evaluated and the role of combination therapy must be further characterized. As the pathogenesis of CD becomes better understood at the molecular level, it is likely that novel, targeted medical therapies will be developed to treat CD.

Similar content being viewed by others
Abbreviations
- ACTH:
-
Adrenocorticotropin
- CD:
-
Cushing’s disease
- CRH:
-
Corticotropin releasing hormone
- CS:
-
Cushing’s syndrome
- RXR:
-
Retinoid acid receptor X
- TSS:
-
Transsphenoidal surgery
- UFC:
-
Urine free cortisol
References
Cushing H (1932) The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp 50:137–195
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367(9522):1605–1617
Tritos NA, Biller BM, Swearingen B (2011) Management of Cushing disease. Nat Rev Endocrinol 7(5):279–289
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93(7):2454–2462
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93(5):1526–1540
Barker FG 2nd, Klibanski A, Swearingen B (2003) Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab 88(10):4709–4719
Swearingen B, Biller BM, Barker FG 2nd, Katznelson L, Grinspoon S, Klibanski A, Zervas NT (1999) Long-term mortality after transsphenoidal surgery for Cushing disease. Ann Intern Med 130(10):821–824
Blevins LS Jr, Christy JH, Khajavi M, Tindall GT (1998) Outcomes of therapy for Cushing’s disease due to adrenocorticotropin-secreting pituitary macroadenomas. J Clin Endocrinol Metab 83(1):63–67
Bochicchio D, Losa M, Buchfelder M (1995) Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab 80(11):3114–3120
Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF (2002) Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 56(4):541–551
Shimon I, Ram Z, Cohen ZR, Hadani M (2002) Transsphenoidal surgery for Cushing’s disease: endocrinological follow-up monitoring of 82 patients. Neurosurgery 51(1):57–61 (discussion 61–52)
Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, Laws ER Jr (2008) Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab 93(2):358–362
Sonino N, Zielezny M, Fava GA, Fallo F, Boscaro M (1996) Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J Clin Endocrinol Metab 81(7):2647–2652
Chen JC, Amar AP, Choi S, Singer P, Couldwell WT, Weiss MH (2003) Transsphenoidal microsurgical treatment of Cushing disease: postoperative assessment of surgical efficacy by application of an overnight low-dose dexamethasone suppression test. J Neurosurg 98(5):967–973
Estrada J, Garcia-Uria J, Lamas C, Alfaro J, Lucas T, Diez S, Salto L, Barcelo B (2001) The complete normalization of the adrenocortical function as the criterion of cure after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 86(12):5695–5699
Trainer PJ, Lawrie HS, Verhelst J, Howlett TA, Lowe DG, Grossman AB, Savage MO, Afshar F, Besser GM (1993) Transsphenoidal resection in Cushing’s disease: undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol (Oxf) 38(1):73–78
Bertagna X, Guignat L (2013) Approach to the Cushing’s disease patient with persistent/recurrent hypercortisolism after pituitary surgery. J Clin Endocrinol Metab 98(4):1307–1318
Liubinas SV, Porto LD, Kaye AH (2011) Management of recurrent Cushing’s disease. J Clin Neurosci 18(1):7–12
Estrada J, Boronat M, Mielgo M, Magallon R, Millan I, Diez S, Lucas T, Barcelo B (1997) The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N Engl J Med 336(3):172–177
Petit JH, Biller BM, Yock TI, Swearingen B, Coen JJ, Chapman P, Ancukiewicz M, Bussiere M, Klibanski A, Loeffler JS (2008) Proton stereotactic radiotherapy for persistent adrenocorticotropin-producing adenomas. J Clin Endocrinol Metab 93(2):393–399
Vella A, Thompson GB, Grant CS, van Heerden JA, Farley DR, Young WF Jr (2001) Laparoscopic adrenalectomy for adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 86(4):1596–1599
Nelson DH, Meakin JW, Dealy JB Jr, Matson DD, Emerson K Jr, Thorn GW (1958) ACTH-producing tumor of the pituitary gland. N Engl J Med 259(4):161–164
Tritos NA, Schaefer PW, Stein TD (2011) Case records of the Massachusetts General Hospital. Case 40-2011. A 52-year-old man with weakness, infections, and enlarged adrenal glands. N Engl J Med 365(26):2520–2530
Assie G, Bahurel H, Coste J, Silvera S, Kujas M, Dugue MA, Karray F, Dousset B, Bertherat J, Legmann P, Bertagna X (2007) Corticotroph tumor progression after adrenalectomy in Cushing’s disease: a reappraisal of Nelson’s syndrome. J Clin Endocrinol Metab 92(1):172–179
Chow JT, Thompson GB, Grant CS, Farley DR, Richards ML, Young WF Jr (2008) Bilateral laparoscopic adrenalectomy for corticotrophin-dependent Cushing’s syndrome: a review of the Mayo clinic experience. Clin Endocrinol (Oxf) 68(4):513–519
Tritos NA, Biller BM (2012) Advances in medical therapies for Cushing’s syndrome. Discov Med 13(69):171–179
Findling JW, Raff H (2006) Cushing’s syndrome: important issues in diagnosis and management. J Clin Endocrinol Metab 91(10):3746–3753
Klibanski A (2010) Clinical practice prolactinomas. N Engl J Med 362(13):1219–1226
de Bruin C, Feelders RA, Lamberts SW, Hofland LJ (2009) Somatostatin and dopamine receptors as targets for medical treatment of Cushing’s syndrome. Rev Endocr Metab Disord 10(2):91–102
Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94(1):223–230
Godbout A, Manavela M, Danilowicz K, Beauregard H, Bruno OD, Lacroix A (2010) Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur J Endocrinol 163(5):709–716
Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (2007) Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 356(1):29–38
Valassi E, Klibanski A, Biller BM (2010) Clinical review: potential cardiac valve effects of dopamine agonists in hyperprolactinemia. J Clin Endocrinol Metab 95(3):1025–1033
Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G (2007) Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med 356(1):39–46
Nieman LK (2013) Update in the medical therapy of Cushing’s disease. Curr Opin Endocrinol Diabetes Obes 20(4):330–334
Ben-Shlomo A, Schmid H, Wawrowsky K, Pichurin O, Hubina E, Chesnokova V, Liu NA, Culler M, Melmed S (2009) Differential ligand-mediated pituitary somatostatin receptor subtype signaling: implications for corticotroph tumor therapy. J Clin Endocrinol Metab 94(11):4342–4350
Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, Reincke M, Snyder P, Tabarin A, Biller BM, Findling J, Melmed S, Darby CH, Hu K, Wang Y, Freda PU, Grossman AB, Frohman LA, Bertherat J (2009) Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94(1):115–122
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM (2012) A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med 366(10):914–924
Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S (2013) Hyperglycemia associated with pasireotide:results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. doi:10.1210/jc.2013-1771
Engelhardt D, Weber MM (1994) Therapy of Cushing’s syndrome with steroid biosynthesis inhibitors. J Steroid Biochem Mol Biol 49(4–6):261–267
McCance DR, Ritchie CM, Sheridan B, Atkinson AB (1987) Acute hypoadrenalism and hepatotoxicity after treatment with ketoconazole. Lancet 1(8532):573
Verhelst JA, Trainer PJ, Howlett TA, Perry L, Rees LH, Grossman AB, Wass JA, Besser GM (1991) Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing’s syndrome. Clin Endocrinol (Oxf) 35(2):169–178
Luton JP, Mahoudeau JA, Bouchard P, Thieblot P, Hautecouverture M, Simon D, Laudat MH, Touitou Y, Bricaire H (1979) Treatment of Cushing’s disease by O, p’DDD. Survey of 62 cases. N Engl J Med 300(9):459–464
Baudry C, Coste J, Bou Khalil R, Silvera S, Guignat L, Guibourdenche J, Abbas H, Legmann P, Bertagna X, Bertherat J (2012) Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol 167(4):473–481
Kamenicky P, Droumaguet C, Salenave S, Blanchard A, Jublanc C, Gautier JF, Brailly-Tabard S, Leboulleux S, Schlumberger M, Baudin E, Chanson P, Young J (2011) Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab 96(9):2796–2804
Schulte HM, Benker G, Reinwein D, Sippell WG, Allolio B (1990) Infusion of low dose etomidate: correction of hypercortisolemia in patients with Cushing’s syndrome and dose-response relationship in normal subjects. J Clin Endocrinol Metab 70(5):1426–1430
Fleseriu M, Molitch ME, Gross C, Schteingart DE, Vaughan TB, Biller BM (2013) A new therapeutic approach in the medical treatment of Cushing’s syndrome: glucocorticoid receptor blockade with Mifepristone. Endocr Pract 19(2):313–326
Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C (2012) Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab 97(6):2039–2049
Feelders RA, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, Zelissen PM, van Heerebeek R, de Jong FH, van der Lely AJ, de Herder WW, Hofland LJ, Lamberts SW (2010) Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N Engl J Med 362(19):1846–1848
Vilar L, Naves LA, Azevedo MF, Arruda MJ, Arahata CM, Moura ESL, Agra R, Pontes L, Montenegro L, Albuquerque JL, Canadas V (2010) Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary 13(2):123–129
Acknowledgments
Dr. Tritos has served as the principal investigator of research sponsored by Pfizer and Ipsen, has consulted for Pfizer and participated in scientific advisory board meetings organized by Pfizer and Corcept Therapeutics. Dr. Biller has served as the principal investigator of Grants to the MGH Neuroendocrine Clinical Center from Novartis and Corcept and as an occasional consultant to Novartis and HRA Pharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tritos, N.A., Biller, B.M.K. Medical management of Cushing’s disease. J Neurooncol 117, 407–414 (2014). https://doi.org/10.1007/s11060-013-1269-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1269-1