Abstract
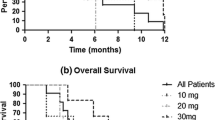
Temozolomide is known to penetrate the blood–brain barrier and sensitize brain tumors to radiation and has been used clinically to sensitize fractionated external beam radiotherapy. However, there are limited prospective clinical data available on the safety of temozolomide as a chemosensitizing agent administered with stereotactic radiosurgery. This is a phase I trial of previously irradiated patients with one to four progressive brain metastases and Karnofsky performance scale score ≥60 % enrolled in three sequential cohorts: temozolomide 100, 150 or 200 mg/(m2 day) administered for 5 days. Stereotactic radiosurgery (SRS) was administered on day 5. The SRS dose was dependent on target diameter: 15 Gy (31–40 mm), 18 Gy (21–30 mm) or 21 Gy (<20 mm). The primary endpoint was safety of increasing temozolomide doses. Secondary endpoints included local control and survival. 26 subjects were enrolled and 49 total metastatic lesions were treated. The median number of brain metastases was 1.5, with a median target diameter of 21 mm. The most common grade 1–2 adverse events irrespective of causality were vomiting (23 %), nausea (23 %), edema (12 %), seizure (8 %), psychosis (4 %) and thrombocytopenia (4 %). The frequency of nausea and vomiting did not appear to be dose-dependent. Grade 3–4 toxicities were not observed. Median overall survival was 10.2 months. Crude local control was 87.5 %, with a radiological response seen in eight of 24 evaluable patients (33.3 %), and stable disease >6 months in 13 of 24 patients (54.2 %). Temozolomide, at doses up to 200 mg/(m2 day) × 5 days, prior to SRS is well tolerated, with no dose-limiting toxicities in patients with recurrent brain metastases. Local control of target lesions was >80 %.

Similar content being viewed by others
References
Easaw JC, Mason WP, Perry J et al (2011) Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol 18:e126–e1362
Mason WP, Maestro RD, Eisenstat D et al (2007) Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol 14:110–117
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5 year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Bull VL, Tisdale MJ (1987) Antitumour imidazotetrazines––XVI. Macromolecular alkylation by 3-substituted imidazotetrazinones. Biochem Pharmacol 36:3215–3220
Pegg AE, Dolan ME, Moschel RC (1995) Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol 51:167–223
Geiger GA, Fu W, Kao GD (2008) Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res 68:3396–3404
Kil WJ, Cerna D, Burgan WE et al (2008) In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res 14:931–938
Tolcher AW, Gerson SL, Denis L et al (2003) Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer 88:1004–1011
Addeo R, Caraglia M, Faiola V et al (2007) Concomitant treatment of brain metastasis with whole brain radiotherapy [WBRT] and temozolomide [TMZ] is active and improves quality of life. BMC Cancer 7:18
Addeo R, De Rosa C, Faiola V et al (2008) Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer 113:2524–2531
Antonadou D, Paraskevaidis M, Sarris G et al (2002) Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol 20:3644–3650
Atkins MB, Sosman JA, Agarwala S et al (2008) Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II cytokine working group study. Cancer 113:2139–2145
Chua D, Krzakowski M, Chouaid C et al (2010) Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-small-cell lung cancer: a randomized, open-label phase II study. Clin Lung Cancer 11:176–181
Cortot AB, Geriniere L, Robinet G et al (2006) Phase II trial of temozolomide and cisplatin followed by whole brain radiotherapy in non-small-cell lung cancer patients with brain metastases: a GLOT–GFPC study. Ann Oncol 17:1412–1417
Giorgio CG, Giuffrida D, Pappalardo A et al (2005) Oral temozolomide in heavily pre-treated brain metastases from non-small cell lung cancer: phase II study. Lung Cancer 50:247–254
Hofmann M, Kiecker F, Wurm R et al (2006) Temozolomide with or without radiotherapy in melanoma with unresectable brain metastases. J Neurooncol 76:59–64
Hwu WJ, Lis E, Menell JH et al (2005) Temozolomide plus thalidomide in patients with brain metastases from melanoma: a phase II study. Cancer 103:2590–2597
Iwamoto FM, Omuro AM, Raizer JJ et al (2008) A phase II trial of vinorelbine and intensive temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol 87:85–90
Kouvaris JR, Miliadou A, Kouloulias VE et al (2007) Phase II study of temozolomide and concomitant whole-brain radiotherapy in patients with brain metastases from solid tumors. Onkologie 30:361–366
Verger E, Gil M, Yaya R et al (2005) Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys 61:185–191
Conti A, Pontoriero A, Arpa D et al (2012) Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir 154:203–209
Balducci M, Apicella G, Manfrida S et al (2010) Single-arm phase II study of conformal radiation therapy and temozolomide plus fractionated stereotactic conformal boost in high-grade gliomas: final report. Strahlenther Onkol 186:558–564
Bobola MS, Kolstoe DD, Blank A et al (2010) Minimally cytotoxic doses of temozolomide produce radiosensitization in human glioblastoma cells regardless of MGMT expression. Mol Cancer Ther 9:1208–1218
Chalmers AJ, Ruff EM, Martindale C et al (2009) Cytotoxic effects of temozolomide and radiation are additive- and schedule-dependent. Int J Radiat Oncol Biol Phys 75:1511–1519
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Shaw E, Scott C, Souhami L et al (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291–298
Dea N, Borduas M, Kenny B et al (2010) Safety and efficacy of Gamma Knife surgery for brain metastases in eloquent locations. J Neurosurg 113(Suppl):79–83
Davey P, O’Brien PF, Schwartz ML et al (1994) A phase I/II study of salvage radiosurgery in the treatment of recurrent brain metastases. Br J Neurosurg 8:717–723
Hoffman R, Sneed PK, McDermott MW et al (2001) Radiosurgery for brain metastases from primary lung carcinoma. Cancer J 7:121–131
Vogelbaum MA, Angelov L, Lee SY et al (2006) Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg 104:907–912
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Gonzalez-Gomez P, Bello MJ, Alonso ME et al (2004) Promoter methylation status of multiple genes in brain metastases of solid tumors. Int J Mol Med 13:93–98
Ingold B, Schraml P, Heppner FL et al (2009) Homogeneous MGMT immunoreactivity correlates with an unmethylated MGMT promoter status in brain metastases of various solid tumors. PLoS ONE 4:e4775
Wu PF, Kuo KT, Kuo LT et al (2010) O(6)-Methylguanine-DNA methyltransferase expression and prognostic value in brain metastases of lung cancers. Lung Cancer 68:484–490
Olson JJ, Paleologos NA, Gaspar LE et al (2010) The role of emerging and investigational therapies for metastatic brain tumors: a systematic review and evidence-based clinical practice guideline of selected topics. J Neurooncol 96:115–142
Fink J, Born D, Chamberlain MC (2011) Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol 12:240–252
Sanghera P, Perry J, Sahgal A et al (2010) Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 37:36–42
Conflict of interest
David Roberge MD, Luis Souhami MD and Marie-Andrée Fortin, have received research funding for the conduct of this clinical trial. David Roberge and Luis Souhami, have served on advisory boards for Schering/Merck. Jean-François Pouliot PhD, is employed by Merck––the manufacturer of the trial drug.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roberge, D., Souhami, L., Fortin, MA. et al. Chemosensitized radiosurgery for recurrent brain metastases. J Neurooncol 110, 265–270 (2012). https://doi.org/10.1007/s11060-012-0965-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0965-6