Abstract
The performed experiments showed that continuous microwave-assisted polyol synthesis at about 70 °C is a viable process to give silver nanoparticles with a narrow size distribution in bulk amounts. The increase of silver precursor concentration in reaction system from 0.0097 to 0.0389 M resulted in the formation of fine nanoparticles 5–10 nm in size, in addition to the larger ones 30–100 nm, and that changed the pattern of particle size distribution from monomodal to bimodal. The effect of specific process variables was found to be the same in the continuous process as in batch operation. An aqueous glycerol solution was found suitable for use as both solvent and reducing agent.
Similar content being viewed by others
Introduction
In the past two decades scores of articles dealt with the preparation of metal nanoparticles (MNP) owing to their unique physical, chemical, and biological properties and exceptional application prospects (Rai et al. 2009; Martinez-Castanon et al. 2008; Dzido et al. 2008; Cho et al. 2005; Buongiorno et al. 2009; Sun and Xia 2002; Filippo et al. 2009; Manoth et al. 2009). Diverse methods for the synthesis of MNPs have been proposed, e.g., microemulsion and reverse micelles (Zhang et al. 2008; Xie et al. 2006), UV-photolysis (Loginov et al. 2002), thermal and sonochemical decomposition (Mourdikoudis et al. 2009; Zhang et al. 2004); electrochemical synthesis (Khaydarov et al. 2009; Starowicz et al. 2006) and chemical reduction with polyols (Gao et al. 2005; Jiang et al. 2006; Li and Komarneni 2006; Zhua et al. 2004; Tsuji et al. 2005; Bhattacharyya and Gedanken 2008). The latter approach that employs polyhydroxy alcohols or their water solutions, acting as both solvent and reducing agent, is particularly suitable for the efficient preparation of easily reducible, uniform mono- and bi-metallic MNP of considerable importance such as Ag, Au, Pt, Pd, and Cu. However, compared with aqueous systems the rate of chemical reduction with polyols is low; it takes many hours or even days, and hence the process needs to be enhanced to render it practical. It was achieved by a microwave (MW) irradiation of the reaction system first proposed by Komarneni et al. (1995) and later adopted by other groups (Jiang et al. 2006; Pastoriza-Santos and Liz-Marzan 2002; Tsuji et al. 2005). The MW irradiation is known for producing very rapid, uniform heating of the solution (thermal effects) that accelerates nucleation of crystals and decelerates its growth due to intense oscillations related particles abrasion (Zhua et al. 2004). Moreover, it also creates hot spots on the solid–liquid surfaces (non-thermal effects) which induce more homogeneous nucleation of metal clusters than in convective heating. In effect, the MW approach appeared to give within minutes metal particles with smaller sizes and narrower size distributions than received in the conventional thermal process (Komarneni et al. 1995; Jiang et al. 2006; Tsuji et al. 2005).
The bulk of reported synthesis was carried out in a batch mode. It is neither particularly efficient nor suitable for a large-quantity production of MNPs in a cost-effective way owing to limited microwave penetration depth which is only a few centimeters long (Stadler et al. 2003). Repeatability of products’ quality achieved from rapid batch processes may sometimes also be a problem (Wu and Zeng 2005). In addition, because of the limited penetration of microwaves into absorbing liquid the processes involving MW heating are difficult to scale-up and continuous flow approach may again appear a viable solution (Glasnov and Kappe 2007). Reports on continuous processes for MNPs are scarce. In fact we are familiar with only one article dealing with a truly continuous flow MW-assisted fabrication of metal particles in a polyol process (Tu and Liu 2000) and another one in which the performance of circulating continuous flow microwave system was extensively examined to show its technical viability and considerable advantages (Groisman and Gedenken 2008). But owing to full recycle of reagents and products the later process was still periodic. In view of the aforementioned we deem it important to shed more light on characteristics and potentials of the continuous fabrication of MNPs using polyol process and it was the prime goal of this work.
In this article, we report the results of studies of the continuous synthesis of silver NP in a typical set-up, using the most conventional reaction system: silver acetate as Ag precursor, ethylene glycol (EG) as both solvent and reducing agent, and polyvinylpyrrolidone (PVP) as a capping and stabilizing agent. Both composition and reaction parameters were varied and properties of the nanoparticles obtained were compared with those synthesized under similar conditions but in a batch approach. In addition, we also checked the potentials of 60 vol% aqueous solution of glycerol (ASG) instead of EG, to drive the process under study toward a greenish synthesis.
Experimental
Materials
Silver acetate (+99%, POCH SA, Poland) was used as silver precursor and ethylene glycol (+99%) or glycerol (+99.5, both POCH, Poland) as both solvent and reducing agent. PVP: K-25, K-30, and K-90 (extra pure, ROTH) with mean MW of 24, 40, and 360 kDa, respectively, was used as capping and stabilizing agent. All chemicals were used as obtained.
Experimental set-up
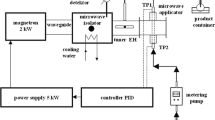
Schematic view of the custom-made apparatus used for the MW-assisted synthesis of MNP is shown in Fig. 1. A domestic microwave oven (Panasonic, 800 W power output) was modified by installing in its cavity (2) a reactor coil (3) made of borosilicate glass tube (8 mm i.d., tube length 2.3 m). Reaction mixture was pumped by a metering pump (8) from a storage vessel (1) into reactor (3) and then through a water cooler (6) to a collecting vessel (7). Inlet and outlet temperature was measured by K-type thermocouples located externally in thermometric nests (5) and connected by A/D converter to the computer. Microwave oven energy was delivered discontinuously (pulsed mode) and its output was controlled by changing on/off time ratio.
Synthesis of silver nanoparticles
To prepare the reaction solution, first a specified amount of PVP, determined to give molar ratio Ag:PVP (monomeric unit) 1:7 or 1:15, was dissolved in ethylene glycol (typically 1 L) at 80 °C under magnetic stirring to obtain a clear solution that was cooled down to room temperature. Then a specified amount of silver acetate, AgAc, determined to give concentration in reaction solution of 0.0097 or 0.0389 M, was dissolved in a tenth of that solution under ultrasonic stirring (at reduced power to avoid overheating) for 5 min., after which it was magnetically homogenized with the remaining part of the PVP–glycol solution to obtain a clear liquid. Afterward, a substrate container was connected to the microwave reactor system. Synthesis was conducted at about 70 °C (outlet temperature) to ensure energy efficiency. Residence time was varied in the range of about 14–28 min by changing the flow rate in the range of 0.25–0.50 L/h. After stable outlet temperature was reached (ca. 70 ± 5 °C) the discharged dispersion was sampled for further characterization. For comparison, a number of batch tests were carried out for identical reaction systems, with aliquot volume same as reactor coil and both residence time and thermal conditions similar to the continuous process.
Characterization
Size of the nanoparticles was determined by a dynamic light scattering technique using a Zetasizer S90 instrument (Malvern Instrument, UK). UV–Vis absorption spectra were recorded using a Hitachi U-2800A spectrophotometer with quartz cuvette. HRTEM morphological analysis of MNPs was performed with a JEOL 3010 field emission microscope working with accelerating voltage of 300 kV. First a sample of silver nanoparticles suspension was centrifuged at 15000 l/min for 30 min. Supernatant liquid was removed and the remain was washed with pure water and again centrifuged. After dilution a drop of colloid was placed on a holey carbon film covering copper grid and allowed to evaporate at room temperature.
Results and discussion
Overall performance of the continuous reaction system
Preliminary experiments showed that smooth operation of the continuous production of Ag NPs is not that easy and the applied flow rate had to be carefully adjusted to the supplied power. As result of too high flow rate low temperature of the reaction liquid was approached and hence the reaction could not run in a microwave cavity. Otherwise, too low flow rates led to overheating (boiling) of the host liquid and even its uncontrolled discharge. Suitable flow rates allowed to keep the region of colloidal system formation inside microwave cavity and its migration with the change in flow rate could easily be seen from the change in color of the reaction liquid from colorless into brown. In that way we could conveniently control the process. But to make sure that silver nanoparticles were indeed synthesized we checked UV-absorption spectra of the colloidal systems. At nominal power input of 100 W (3 s on/17 s off) the values of operational flow rates were found to be approximately between 0.25 and 0.50 L/h and hence the bulk of experiments were performed for those values. But clearly, the very complex relationships between kinetics of metal colloids formation and aggregation, process variables and final properties of nanocolloids obtained leaves a huge room for optimization of the whole system. It was yet considered beyond the scope of this work.
Impact of synthesis parameters
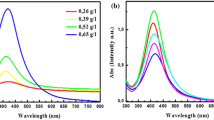
All UV–Vis absorption spectra of the colloidal systems obtained from the reactions liquids (ASG included) listed in Table 1, regardless the fabrication mode, showed very pronounced single absorption band with maximum around 415–435 nm assigned by Mie’s theory to the surface plasmon resonance from small spherical silver nanocrystals (Petit et al. 1993; Bonnemann et al. 2005). The peaks were symmetrical and narrow for all systems (cf. Figs. 2, 3, 4), evidencing a fair similarity in the shape of particles. But loci of maximum of UV-absorption bands differed considerably (cf. Table 1), depending more on the composition of the reaction liquid and less on reaction time. This indicated that both size and surrounding of the particles depend on the reaction conditions in a complex way (Kundu et al. 2009).
On the whole the shifts in UV–Vis absorption bands and the corresponding particle size distribution (PSD) histograms determined using a DLS method consistently indicated major impacts of process parameters on particle sizes but failed to be in full agreement in more detailed studies. More specifically, the results given in Table 1 show that the use of PVPs with smaller repeat units (K25, K30) resulted in smaller particles than of the larger one (K90) and also that increase of the molar PVP: metal precursor value from 7 to 15 had little effect on the particle size. This is in good agreement with the earlier reports that PVP with low molecular weight more swiftly and efficiently prevented agglomeration and hampered particle growth and also that above a threshold value of PVP content its further increase had no appreciable effect on the particle size (Jiang et al. 2006; Zhua et al. 2004; Tsuji et al. 2005). This is consistently indicated by both blue-shifts in absorption bands and PSDs obtained from samples with larger amount of the same PVP (cf. Table 1) and red-shifts in UV-absorption bands from samples 1, 3, and 5 obtained utilizing PVPs with progressively longer chains but in the same amount (cf. Fig. 4). Yet the shifts in spectra from similar samples but fabricated at larger flow rates were less consistent in that respect (cf. Table 1). Therefore, these shifts are hardly a sole indicative of particle sizes but also in their chemical surrounding. Indeed, the average size of particles obtained using longer chain PVP (K90, sample 5) determined by DLS measurements and also TEM (not shown here) appeared to well exceed 100 nm and hence it was significantly larger than for K30 (ca. 60 nm, cf. Table 1), in agreement with earlier observations of Tsuji et al. (2005) but not quite in agreement with the predictions from UV-absorption analysis (Table 1).
Surprisingly, an application of larger initial concentration of AgAc (0.0389 M) had a significant effect on the particle sizes (Table 1, cf. samples 1 with 6 and 3 with 7). Its fourfold increase resulted in blue-shifted absorption peaks (from 420–430 to 415–420 nm) as can be seen from Fig. 2 and Table 1, implying a significant decrease in average particle size. This trend of particle size reduction was also confirmed by DLS measurements. But even more surprisingly, the latter also clearly evidenced a bi-modal size distribution pattern of particles in samples 6 and 7, with numerous very small particles of few nm in size present in addition to the larger ones from the region of 30–100 nm (cf. Fig. 5). The origin and mechanism of formation of these very small nanoparticles is not quite clear. Typically larger concentrations of metal precursors in reaction systems were observed to give larger nanoparticles (Jiang et al. 2006; Tsuji et al. 2005). We may speculate that oscillations related particles abrasion, more intense at higher concentrations, under slow rate of AgAc reduction at low temperature, induced their formation and rapid growth (Zhua et al. 2004). But more detailed investigations of that are necessary to elucidate the problem. Interestingly enough, these very small nanoparticles were also clearly detected by a DLS method when the aqueous solution of glycerol (sample 8) was applied instead of EG, even though the concentration of silver precursor was low (Fig. 6). Although, both families of particles were notably larger in that case (cf. Figs. 5, 6), the mechanisms of their formation were most likely quite similar. But more importantly, the latter results also show that solvent has a significant effect on the rate of the process and specific properties of the particles.
Under the given reaction conditions the extension of mean residence (reaction) time in an oven in the range of 14–28 min had less effect on particles sizes than other experimental parameters discussed above. At lower initial AgAc concentration the size of particles produced during the prolonged process was about 10% smaller than in the shorter one, but at larger concentration (0.0389 M) an opposite trend was observed. Those trends were fairly consistently indicated by both DLS measurements and the shifts in UV–Visible absorption spectra (Table 1).
An analysis of TEM images indicated that most of nanoparticles obtained from the process were spherical (Fig. 7) and that can be linked to the conditions of their formations. But larger particles of irregular shape, presumably originating from the aggregation process, could also be seen. The NPs obtained tended to be polycrystalline, some of them twinned (cf. Fig. 8), but no prevalent morphology has been determined as SAED patterns were not clear enough.
Conclusions
The experiments performed clearly indicate that silver nanoparticles, and metal nanoparticles in general, can efficiently be produced in a continuous low-temperature MW-assisted polyol process and that the effect of various process parameters and reaction variables is much the same as observed or reported for the corresponding batch operations, carried out under similar conditions. This observation paves the way to a wider application of the continuous processes of MNPs production using the experience and knowledge accumulated from the operation of batch systems. Silver nanoparticles can be produced in a continuous polyol process at temperatures as low as 70 °C using ethylene glycol or an aqueous glycerol solution as a solvent and reducing agent.
Under the given synthesis conditions a slight increase in the concentration of silver precursor appeared to result in the formation of numerous very small nanoparticles, in addition to the larger ones, and that changed the particle size distribution pattern from mono- to bi-modal.
References
Bhattacharyya S, Gedanken A (2008) Microwave-assisted insertion of silver nanoparticles into 3-D mesoporous zinc oxide nanocomposites and nanorods. J Phys Chem C 112:659–665. doi:10.1021/jp0760253
Bonnemann H, Botha SS, Bladergroen B, Linkov VM (2005) Monodisperse copper- and silver-nanocolloids suitable for heat-conductive fluids. Appl Organomet Chem 19:768–773. doi:10.1002/aoc.889
Buongiorno J, Venerus DC, Dzido G, Jarzebski AB et al (2009) A benchmark study on the thermal conductivity of nanofluids. J Appl Phys 106:094312. doi:10.1063/1.3245330
Cho T, Baek I, Lee J, Park S (2005) Preparation of nanofluid containing suspended silver particles for enhancing fluid thermal conductivity of fluids. J Ind Eng Chem 11(3):400–406
Dzido G, Chmiel-Kurowska K, Gierczycki A, Jarzębski AB (2008) Convective heat transfer in Ag– and Cu–ethylene glycol nanofluids. NSTI Nanotechnology Conference, Boston, 2008. Tech Proc 1:778–781
Filippo E, Serra A, Manno D (2009) Poly(vinyl alcohol) capped silver nanoparticles as localized surface plasmon resonance-based hydrogen peroxide sensor. Sens Actuators B 138:625–630. doi:10.1016/j.snb.2009.02.056
Gao F, Lu Q, Komarneni S (2005) Interface reaction for the self-assembly of silver nanocrystals under microwave-assisted solvothermal conditions. Chem Mater 17:856–860. doi:10.1021/cm048663t
Glasnov TN, Kappe CO (2007) Microwave-assisted synthesis under continuous-flow conditions. Macromol Rapid Commun 28:395–410. doi:10.1002/marc.200600665
Groisman Y, Gedenken A (2008) Continuous flow, circulating microwave system and its application in nanoparticle fabrication and biodiesel synthesis. J Phys Chem C 112:8802–8808. doi:10.1021/jp801409t
Jiang H, Moon K, Zhang Z, Pothukuchi S, Wong CP (2006) Variable frequency microwave synthesis of silver nanoparticles. J Nanopart Res 8:117–124. doi:10.1007/s11051-005-7522-6
Khaydarov RA, Khaydarov RR, Gapurova O, Estrin Y, Scheper T (2009) Electrochemical method for the synthesis of silver nanoparticles. J Nanopart Res 11:1193–1200. doi:10.1007/s11051-008-9513-x
Komarneni S, Pidugu R, Li QH, Roy R (1995) Microwave-hydrothermal processing of metal powders. J Mater Res 10:1687–1692
Kundu S, Wang K, Liang H (2009) Size-controlled synthesis and self-assembly of silver nanoparticles within a minute using microwave irradiation. J Phys Chem C 113:134–141. doi:10.1021/jp808292s
Li D, Komarneni S (2006) Microwave-assisted polyol process for synthesis of Ni nanoparticles. J Am Ceram Soc 89:1510–1517. doi:10.1111/j1551-2916.2006
Loginov AV, Gorbunova VV, Boitsova TB (2002) Photochemical synthesis and properties of colloidal copper, silver and gold adsorbed on quartz. J Nanopart Res 4:193–205. doi:10.1023/A:1019966021598
Manoth M, Manzoor K, Patra MK, Pandey P, Vadera SR, Kumar N (2009) Dendrigraft polymer-based synthesis of silver nanoparticles showing bright blue fluorescence. Mat Res Bull 44:714–717. doi:10.1016/j.materresbull.2008.06.033
Martinez-Castanon GA, Ninio-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza JR, Ruiz F (2008) Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res 10:1343–1348. doi:10.1007/s11051-008-9428-6
Mourdikoudis S, Simeonidis K, Tsiaoussis I, Dendrinou-Samara C, Angelakeris M, Kalogriou O (2009) Impact of synthesis parameters on structural and magnetic characteristics of Co-based nanoparticles. J Nanopart Res 11:1477–1484. doi:10.1007/s11051-008-9428-6
Pastoriza-Santos I, Liz-Marzan LM (2002) Formation of PVP-protected metal nanoparticles in DMF. Langmuir 18:2888–2894. doi:10.1021/la015578g
Petit Ch, Lixonf P, Pileni MP (1993) In situ synthesis of silver nanocluster in AOT reverse micelles. J Phys Chem 97:12974–12983. doi:10.1021/j100151a054
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. doi:10.1016/j.biotechadv.2008.09.002
Stadler A, Yousefi BH, Dallinger D, Walla P, Eycken E, Kaval N, Kappe CO (2003) Scalability of microwave–assisted organic synthesis. From single- mode to multimode parallel batch reactors. Org Proc Res Dev 7:707–716. doi:10.1021/op034075+
Starowicz M, Stypuła B, Banaś J (2006) Electrochemical synthesis of silver nanoparticles. Electrochem Commun 8:227–230. doi:10.1016/j.elecom.2005.11.018
Sun Y, Xia Y (2002) Large-scale synthesis of uniform silver nanowires through a soft, self-ending, polyol process. Adv Mater 14(11):833–837
Tsuji M, Hashimoto M, Nishizawa Y, Kubokawa M, Tsuji T (2005) Microwave-assisted synthesis of metallic nanostructures in solution. Chem Eur J 11:440–452. doi:10.1002/chem.200400417
Tu W, Liu H (2000) Continuous synthesis of colloidal metal nanoclusters by microwave irradiation. Chem Mater 12:564–567. doi:10.1021/cm990637l
Wu Ch, Zeng T (2005) Size-tunable synthesis of metallic nanoparticles in a continuous and steady-flow reactor. Chem Mater 19(2):123–125. doi:10.1021/cm062344f
Xie Y, Ye R, Liu H (2006) Synthesis of silver nanoparticles in reverse micelles stabilized by natural biosurfactant. Colloids Surf A Physicochem Eng Aspects 279:175–178
Zhang J, Chen P, Sun Ch, Hu X (2004) Sonochemical synthesis of colloidal silver catalysts for reduction of complexing silver in DTR system. Appl Catal A 266:49–54. doi:10.1016/j.apcata.2004.01.025
Zhang W, Qiao X, Chen J (2008) Formation of silver nanoparticles in SDS inverse microemulsions. Mat Chem Phys 109:411–416. doi:10.1016/j.matchemphys.2007.12.009
Zhua H, Zhang C, Yin Y (2004) Rapid synthesis of copper nanoparticles by sodium hypophosphite reduction in ethylene glycol under microwave irradiation. J Crystal Growth 270:722–728. doi:10.1016/j.jcrysgro.2004.07.008
Acknowledgments
The author gratefully acknowledge the Polish Ministry for Science and Higher Education for support for this work under grant R14 02602. We also appreciate Prof. Jozef Lelatko for TEM images and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dzido, G., Jarzębski, A.B. Fabrication of silver nanoparticles in a continuous flow, low temperature microwave-assisted polyol process. J Nanopart Res 13, 2533–2541 (2011). https://doi.org/10.1007/s11051-010-0146-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0146-5