Abstract
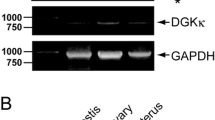
Renalase was initially identified in human kidney as a soluble monoamine oxidase. Here we show that renalase is predominantly expressed in reproductive/steroidogenic systems, with particularly substantial expression in oocytes, granulosa, interstitial and luteal cells of ovary, spermatogenic cells of testis, and cortex of adrenal gland, suggesting its function(s) in maturation of germ cells and steroid hormone regulation. Renalase expression increases in testes and ovaries as mice develop and its expression is further enhanced in the ovaries of pregnant mice, indicating an activity of renalase in reproduction. Gonadotropin-releasing hormone (GnRH) antagonist, cetrorelix, repressed renalase expression in mice ovaries and testes, suggesting that steroids regulate renalase expression. Leptin is an effector and modulator of steroid hormones and reproduction. Surprisingly, knockout of leptin causes a dramatic increase of renalase expression in mice testes. Taken together, our results suggest that reproductive/steroidogenic systems are also the sources for renalase secretion and renalase may play a critical role in reproduction and hormone regulation. This provides a novel insight into understanding the function of renalase.




Similar content being viewed by others
Abbreviations
- NA:
-
Noradrenaline
- ADR:
-
Adrenaline
- DA:
-
Dopamine
- FAD:
-
Flavin adenine dinucleotide
- ESRD:
-
End-stage renal disease
- GnRH:
-
Gonadotropin-releasing hormone
- ob/ob:
-
Leptin knockout
- IHC:
-
Immunohistochemistry
- LH:
-
Luteinizing hormone
- FSH:
-
Follicle-stimulating hormone
- LEPR:
-
Leptin receptor
References
Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV (2005) Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest 115(5):1275–1280. doi:10.1172/JCI24066
Boomsma F, Tipton KF (2007) Renalase, a catecholamine-metabolising enzyme? J Neural Transm 114(6):775–776. doi:10.1007/s00702-007-0672-1
Xu J, Desir GV (2007) Renalase, a new renal hormone: its role in health and disease. Curr Opin Nephrol Hypertens 16(4):373–378. doi:10.1097/MNH.0b013e3281bd8877
Li G, Xu J, Wang P, Velazquez H, Li Y, Wu Y, Desir GV (2008) Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation 117(10):1277–1282. doi:10.1161/CIRCULATIONAHA.107.732032
Desir GV (2007) Renalase is a novel renal hormone that regulates cardiovascular function. J Am Soc Hypertens 1(2):99–103. doi:10.1016/j.jash.2006.12.001
Pandini V, Ciriello F, Tedeschi G, Rossoni G, Zanetti G, Aliverti A (2010) Synthesis of human renalase1 in Escherichia coli and its purification as a FAD-containing holoprotein. Protein Expr Purif 72(2):244–253. doi:10.1016/j.pep.2010.03.008
Milani M, Ciriello F, Baroni S, Pandini V, Canevari G, Bolognesi M, Aliverti A (2011) FAD-binding site and NADP reactivity in human renalase: a new enzyme involved in blood pressure regulation. J Mol Biol 411(2):463–473. doi:10.1016/j.jmb.2011.06.010
Zhao Q, Fan Z, He J, Chen S, Li H, Zhang P, Wang L, Hu D, Huang J, Qiang B, Gu D (2007) Renalase gene is a novel susceptibility gene for essential hypertension: a two-stage association study in northern Han Chinese population. J Mol Med 85(8):877–885. doi:10.1007/s00109-006-0151-4
Wang J, Qi S, Cheng W, Li L, Wang F, Li YZ, Zhang SP (2008) Identification, expression and tissue distribution of a renalase homologue from mouse. Mol Biol Rep 35(4):613–620. doi:10.1007/s11033-007-9131-1
Hennebry SC, Eikelis N, Socratous F, Desir G, Lambert G, Schlaich M (2010) Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry 15(3):234–236. doi:10.1038/mp.2009.74 mp200974 [pii]
Meirow D, Assad G, Dor J, Rabinovici J (2004) The GnRH antagonist cetrorelix reduces cyclophosphamide-induced ovarian follicular destruction in mice. Hum Reprod 19(6):1294–1299. doi:10.1093/humrep/deh257
Di Cristo C, De Lisa E, Di Cosmo A (2009) Control of GnRH expression in the olfactory lobe of Octopus vulgaris. Peptides 30(3):538–544. doi:10.1016/j.peptides.2008.07.007
Madgwick S, Bagu ET, Duggavathi R, Bartlewski PM, Barrett DM, Huchkowsky S, Cook SJ, Beard AP, Rawlings NC (2008) Effects of treatment with GnRH from 4 to 8 weeks of age on the attainment of sexual maturity in bull calves. Anim Reprod Sci 104(2–4):177–188. doi:10.1016/j.anireprosci.2007.02.013
Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM (2006) Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol 149(1):5–13. doi:10.1038/sj.bjp.0706834
Di Carlo C (2003) Leptin and female reproduction. J Endocrinol Invest 26(1):93–95
Holness MJ, Munns MJ, Sugden MC (1999) Current concepts concerning the role of leptin in reproductive function. Mol Cell Endocrinol 157(1–2):11–20
Magni P, Motta M, Martini L (2000) Leptin: a possible link between food intake, energy expenditure, and reproductive function. Regul Pept 92(1–3):51–56
Smith GD, Jackson LM, Foster DL (2002) Leptin regulation of reproductive function and fertility. Theriogenology 57(1):73–86
Chehab FF, Lim ME, Lu R (1996) Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12(3):318–320. doi:10.1038/ng0396-318
Roberts AJ, McLean DJ (2011) Differential gene expression in anterior pituitary glands from anestrous and cycling postpartum beef cows. J Anim Sci 89(4):1035–1041. doi:10.2527/jas.2010-3433
Paulis L, Unger T (2010) Novel therapeutic targets for hypertension. Nat Rev Cardiol 7(8):431–441. doi:10.1038/nrcardio.2010.85
Tandler R, Kondruweit M, Fischlein T, Weyand M (2003) Hormone therapy in men-increased risk of cardiac allograft rejection? J Heart Lung Transplant 22(7):831–832
Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL (1998) Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67(6):370–376
Hausman GJ, Barb CR (2010) Adipose tissue and the reproductive axis: biological aspects. Endocr Dev 19:31–44. doi:10.1159/000316895
Loucks AB (2007) Energy availability and infertility. Curr Opin Endocrinol Diabetes Obes 14(6):470–474. doi:10.1097/MED.0b013e3282f1cb6a01266029-200712000-00009
Barkan D, Hurgin V, Dekel N, Amsterdam A, Rubinstein M (2005) Leptin induces ovulation in GnRH-deficient mice. FASEB J 19(1):133–135. doi:10.1096/fj.04-2271fje
Gueorguiev M, Goth ML, Korbonits M (2001) Leptin and puberty: a review. Pituitary 4(1–2):79–86
Sone M, Osamura RY (2001) Leptin and the pituitary. Pituitary 4(1–2):15–23
Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A (2001) Leptin in reproduction. Trends Endocrinol Metab 12(2):65–72
Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS (1997) Leptin accelerates the onset of puberty in normal female mice. J Clin Invest 99(3):391–395. doi:10.1172/JCI119172
Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG Jr (2011) Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology 152(6):2302–2310. doi:10.1210/en.2011-0096
Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM (2009) Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150(6):2805–2812. doi:10.1210/en.2008-1693
Sairam MR, Wang M, Danilovich N, Javeshghani D, Maysinger D (2006) Early obesity and age-related mimicry of metabolic syndrome in female mice with sex hormonal imbalances. Obesity (Silver Spring) 14(7):1142–1154. doi:10.1038/oby.2006.131
Xu ZP, Wawrousek EF, Piatigorsky J (2002) Transketolase haploinsufficiency reduces adipose tissue and female fertility in mice. Mol Cell Biol 22(17):6142–6147
Chehab FF (1996) A broader role for leptin. Nat Med 2(7):723–724
Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O’Rahilly S (2007) Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356(3):237–247. doi:10.1056/NEJMoa063988
Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM (2011) Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology 152(4):1541–1550. doi:10.1210/en.2010-1100
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL et al (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334(5):292–295. doi:10.1056/NEJM199602013340503
Houseknecht KL, Portocarrero CP (1998) Leptin and its receptors: regulators of whole-body energy homeostasis. Domest Anim Endocrinol 15(6):457–475
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372(6505):425–432. doi:10.1038/372425a0
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395(6704):763–770. doi:10.1038/27376
Acknowledgments
We appreciate the personal communication with Prof. Gary Desir (Yale School of Medicine, USA). We also thank Shaoyong Chen (BIDMC, Harvard Medical School, USA) for reviewing our manuscript and Prof. Peng Li (School of Life Sciences, Tsinghua University, China) for the help with the ob/ob mice tissue.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (81171063, J1030622) and National Key Projects of Ministry of Agriculture of China (2011ZX08011-006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Mingxue Zhou and Tong Liang contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, M., Liang, T., Wang, Y. et al. Expression and tissue localization of renalase, a novel soluble FAD-dependent protein, in reproductive/steroidogenic systems. Mol Biol Rep 40, 3987–3994 (2013). https://doi.org/10.1007/s11033-012-2476-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2476-0