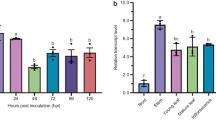
Abstract
Plants have evolved diverse mechanism to recognize pathogen attack and triggers defense responses. These defense responses alter host cellular function regulated by endogenous, small, non-coding miRNAs. To understand the mechanism of miRNAs regulated cellular functions during stem rust infection in wheat, we investigated eight different miRNAs viz. miR159, miR164, miR167, miR171, miR444, miR408, miR1129 and miR1138, involved in three different independent cellular defense response to infection. The investigation reveals that at the initiation of disease, accumulation of miRNAs might be playing a key role in hypersensitive response (HR) from host, which diminishes at the maturation stage. This suggests a possible host-fungal synergistic relation leading to susceptibility. Differential expression of these miRNAs in presence and absence of R gene provides a probable explanation of miRNA regulated R gene mediated independent pathways.




Similar content being viewed by others
References
Yang YN, Shah J, Klessig DF (1997) Signal perception and transduction in plant defense responses. Genes Dev 11:1621–1639
Dangl JL, Dietrich RA, Richberg MH (1996) Death don’t have no mercy: Cell death programs in plant microbe interactions. Plant Cell 8:793–807
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Blumwald E, Aharon GS, Lam BC (1998) Early signal transduction pathway in plant pathogen interactions. Trends Plant Sci 3:342–346
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Ann Rev Plant Biol 57:19–53
Lang Q, Jin CZ, Lai L, Feng J, Chen S, Chen J (2010) Tobacco microRNAs prediction and their expression infected with Cucumber mosaic virus and Potato virus X. Mol Biol Rep. doi:10.1007/s11033-010-0260-6
Gao P, Bai X, Yang L, Lv D, Pan X, Li Y, Cai H, Ji W, Chen Q, Zhu Y (2010) osa-MIR393: a salinity- and alkaline stress-related microRNA gene. Mol Biol Rep. doi:10.1007/s11033-010-0100-8
Liu Q, and Chen YQ (2010) A new mechanism in plant engineering: The potential roles of microRNAs in molecular breeding for crop improvement. Biotechnol Adv. doi:10.1016/j.biotechadv.2010.01.002
Bari R, Jones JDG (2009) Role of plant hormones in plant defense responses. Plant Mol Biol 69:473–488
Guo HS, Xie Q, Fei JF, Chua NH (2005) MicroRNA directs mRNA cleavage of the transcription factor NAC1 to down regulate auxin signals for Arabidopsis lateral root development. Plant Cell 17:1376–1386
Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP (2002) MicroRNAs in plants. Genes Dev 16:1616–1626
Achard P, Herr A, Baulcombe DC, Harberd NP (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131:3357–3365
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of microRNAs on the plant transcriptome. Dev Cell 8:517–527
Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002) Prediction of plant microRNA targets. Cell 110:513–520
Zhang B, Pan X, Anderson TA (2006) Identification of 188 conserved maize microRNAs and their targets. FEBS Letters 580:3753–3762
Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM (2003) Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA 100:16125–16130
Yao Y, Guo G, Ni Z, Sunkar R, Du J, Zhu JK, Sun Q (2007) Cloning and characterization of microRNAs from wheat (Triticum aestivum L.). Genome Biol 8:R96
Yin J, Wang G, Xiao J, Ma F, Zhang H, Sun Y, Diao Y, Huang J, Guo Q, Liu D (2010) Identification of genes involved in stem rust resistance from wheat mutant D51 with the cDNA-AFLP technique. Mol Bio Rep 37:1111–1117
Anikster Y (1984) The formae specials. In: Bushnell WR, Roelfs AP (eds) The cereal rusts, vol 1. Academic Press, New York London, pp 115–129
Seungil Ro, Park C, Jin J, Sanders KM, Yan W (2006) A PCR-based method for detection and quantification of small RNAs. Biochem Biophys Res Commun 351(3):756–763
Stakman EC, and Levine MN (1922) The determination of biologic forms of Puccinia graminis on Triticum spp. Minn Agr Res Stn Bull 8:10
Moerschbacher BM, Noll UM, Flott BE, Reisener HJ (1988) Lignin biosynthetic enzymes in stem rust infected resistant and susceptible near-isogenic wheat lines. Physiol Mol Plant Pathol 33:33–46
Heath MC (1994) Genetics and cytology of age-related resistance in North American cultivars of cowpea (Vigna unguiculata (L.) Walp.) to the cowpea rust fungus (Uromyces vignae Baeclay). Can J Bot 73:575–581
Heath MC (1997) Signaling between pathogenic rust fungi and resistant or susceptible host plants. Ann Bot 80:713–720
Acknowledgments
Om Prakash Gupta was supported by a fellowship from Indian Council of Agricultural Research, New Delhi. Authors are thankful to Ms. Mansi for her help in manuscript preparation. In addition, we thank Mr. C.M. Kushwaha for his technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Om Prakash Gupta and Vipin Permar contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, O.P., Permar, V., Koundal, V. et al. MicroRNA regulated defense responses in Triticum aestivum L. during Puccinia graminis f.sp. tritici infection. Mol Biol Rep 39, 817–824 (2012). https://doi.org/10.1007/s11033-011-0803-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0803-5