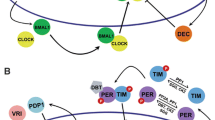
Abstract
Circadian clocks play a fundamental role in biology and disease. Much has been learned about the molecular underpinnings of these biological clocks from genetic studies in model organisms, such as the fruit fly, Drosophila melanogaster. Here we review the literature from our lab and others that establish a role for the protein kinase CK2 in Drosophila clock timing. Among the clock genes described thus far, CK2 is unique in its involvement in plant, fungal, as well as animal circadian clocks. We propose that this reflects an ancient, conserved function for CK2 in circadian clocks. CK2 and other clock genes have been implicated in cellular responses to DNA damage, particularly those induced by ultraviolet (UV) light. The finding of a dual function of CK2 in clocks and in UV responses supports the notion that clocks evolved to assist organisms in avoiding the mutagenic effects of daily sunlight.
Similar content being viewed by others
References
Sehgal A: Molecular biology of circadian rhythms. In: ‘(eds). Wiley, Hoboken, NJ, 2004, pp 283
Plautz JD, Kaneko M, Hall JC, Kay SA: Independent photoreceptive circadian clocks throughout Drosophila [see comments]. Science 278: 1632–1635, 1997
Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H: Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000
Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M: Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001
Lin Y, Han M, Shimada B, Wang L, Gibler TM, Amarakone A, Awad TA, Stormo GD, Van Gelder RN, Taghert PH: Influence of the period-dependent circadian clock on diurnal, circadian, and aperiodic gene expression in Drosophila melanogaster. Proc Natl Acad Sci USA 99: 9562–9567, 2002
Etter PD, Ramaswami M: The ups and downs of daily life: Profiling circadian gene expression in Drosophila. Bioessays 24: 494–498, 2002
McDonald MJ, Rosbash M: Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107: 567–578, 2001
Grundschober C, Delaunay F, Puhlhofer A, Triqueneaux G, Laudet V, Bartfai T, Nef P: Circadian regulation of diverse gene products revealed by mRNA expression profiling of synchronized fibroblasts. J Biol Chem 276: 46751–46758, 2001
Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T: Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5: 18, 2004
Czeisler CA, Klerman EB: Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res 54: 97–132, 1999
Edery I: Circadian rhythms in a nutshell. Physiol Genomics 3: 59–74, 2000
Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH: An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291: 1040–1043, 2001
Fu L, Pelicano H, Liu J, Huang P, Lee C: The circadian gene Period 2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111: 41–50, 2002
Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, Zakany J, Duboule D, Petit B, Tafti M, Schibler U: The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev 18: 1397–1412, 2004
Abe M, Herzog ED, Block GD: Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport 11: 3261–3264, 2000
Klemfuss H: Rhythms and the pharmacology of lithium. Pharmacol Ther 56: 53–78, 1992
Lenox RH, Gould TD, Manji HK: Endophenotypes in bipolar disorder. Am J Med Genet 114: 391–406, 2002
Boivin DB: Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci 25: 446–458, 2000
Lewy AJ, Bauer VK, Cutler NL, Sack RL, Ahmed S, Thomas KH, Blood ML, Jackson JM: Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry 55: 890–896, 1998
Avery DH, Dahl K, Savage MV, Brengelmann GL, Larsen LH, Kenny MA, Eder DN, Vitiello MV, Prinz PN: Circadian temperature and cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression. Biol Psychiatry 41: 1109–1123, 1997
Schwartz WJ, Gainer H: Suprachiasmatic nucleus: Use of 14C-labeled deoxyglucose uptake as a functional marker. Science 197: 1089–1091, 1977
Inouye ST, Kawamura H: Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA 76: 5962–5966, 1979
Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC: In vivo metabolic activity of the suprachiasmatic nuclei: A comparative study. Brain Res 274: 184–187, 1983
Drucker-Colin R, Aguilar-Roblero R, Garcia-Hernandez F, Fernandez-Cancino F, Bermudez Rattoni F: Fetal suprachiasmatic nucleus transplants: Diurnal rhythm recovery of lesioned rats. Brain Res 311: 353–357, 1984
Ralph MR, Foster RG, Davis FC, Menaker M: Transplanted suprachiasmatic nucleus determines circadian period. Science 247: 975–978, 1990
Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP: Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12: 540–550, 2002
Quintero JE, Kuhlman SJ, McMahon DG: The biological clock nucleus: A multiphasic oscillator network regulated by light. J Neurosci 23: 8070–8076, 2003
Ruby NF, Burns DE, Heller HC: Circadian rhythms in the suprachiasmatic nucleus are temperature-compensated and phase-shifted by heat pulses in vitro. J Neurosci 19: 8630–8636, 1999
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U: Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000
Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S: Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6: 269–278, 2001
Kaneko M, Hall JC: Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422: 66–94, 2000
Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE: Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol 14: 638–649, 2004
Ashmore LJ, Sehgal A: A fly’s eye view of circadian entrainment. J Biol Rhythms 18: 206–216, 2003
Oishi K, Shiota M, Sakamoto K, Kasamatsu M, Ishida N: Feeding is not a more potent Zeitgeber than the light-dark cycle in Drosophila. Neuroreport 15: 739–743, 2004
Allada R, Emery P, Takahashi JS, Rosbash M: Stopping time: The genetics of fly and mouse circadian clocks. Annu Rev Neurosci 24: 1091–119, 2001
Konopka RJ, Benzer S: Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 68: 2112–2116, 1971
Rutila JE, Suri V, Le M, So WV, Rosbash M, Hall JC: CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93: 805–814, 1998
Allada R, White NE, So WV, Hall JC, Rosbash M: A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93: 791–804, 1998
Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TDL, Weitz CJ, Takahashi JS, Kay SA: Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 280: 1599–1603, 1998
Blau J, Young MW: Cycling vrille expression is required for a functional Drosophila clock. Cell 99: 661–671, 1999
Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, Young MW, Storti RV, Blau J: Vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112: 329–341, 2003
Glossop NR, Houl JH, Zheng H, Ng FS, Dudek SM, Hardin PE: VRILLE feeds back to control circadian transcription of clock in the Drosophila circadian oscillator. Neuron 37: 249–261, 2003
Allada R: Circadian clocks: A tale of two feedback loops. Cell 112: 284–286, 2003
Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC: The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95: 681–692., 1998
Emery P, So WV, Kaneko M, Hall JC, Rosbash M: CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95: 669–679, 1998
Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, Kay SA: Light-dependent sequestration of timeless by cryptochrome. Science 285: 553–556, 1999
Zhao J, Kilman VL, Keegan KP, Peng Y, Emery P, Rosbash M, Allada R: Drosophila clock can generate ectopic circadian clocks. Cell 113: 755–766, 2003
Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW: Double-time is a novel Drosophila clock gene that regulates period protein accumulation. Cell 94: 83–95, 1998
Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, Young MW: The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94: 97–107, 1998
Martinek S, Inonog S, Manoukian AS, Young MW: A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105: 769–779, 2001
Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, Allada R: A role for casein kinase 2 alpha in the Drosophila circadian clock. Nature 420: 816–820, 2002
Lee C, Bae K, Edery I: The Drosophila CLOCK protein undergoes daily rhythms in abundance, phosphorylation, and interactions with the PER–TIM complex. Neuron 21: 857–867, 1998
Kloss B, Rothenfluh A, Young MW, Saez L: Phosphorylation of period is influenced by cycling physical associations of double-time, period, and timeless in the Drosophila clock. Neuron 30: 699–706, 2001
Bao S, Rihel J, Bjes E, Fan JY, Price JL: The Drosophila double-times mutation delays the nuclear accumulation of period protein and affects the feedback regulation of period mRNA. J Neurosci 21: 7117–7126., 2001
Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS: Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288: 483–492, 2000
Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM: Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol Cell Biol 20: 4888–4899, 2000
Keesler GA, Camacho F, Guo Y, Virshup D, Mondadori C, Yao Z: Phosphorylation and destabilization of human period I clock protein by human casein kinase I epsilon. Neuroreport 11: 951–955, 2000
Price MA, Kalderon D: Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell 108: 823–835, 2002
Picton C, Woodgett J, Hemmings B, Cohen P: Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett 150: 191–196, 1982
Graham KC, Litchfield DW: The regulatory beta subunit of protein kinase CK2 mediates formation of tetrameric CK2 complexes. J Biol Chem 275: 5003–5010, 2000
Gietz RD, Graham KC, Litchfield DW: Interactions between the subunits of casein kinase II. J Biol Chem 270: 13017–13021, 1995
Niefind K, Guerra B, Ermakowa I, Issinger OG: Crystal structure of human protein kinase CK2: Insights into basic properties of the CK2 holoenzyme. Embo J 20: 5320–5331, 2001
Meggio F, Marin O, Pinna LA: Substrate specificity of protein kinase CK2. Cell Mol Biol Res 40: 401–409, 1994
Meggio F, Pinna LA: One-thousand-and-one substrates of protein kinase CK2? Faseb J 17: 349–368, 2003
Sarno S, Ghisellini P, Pinna LA: Unique activation mechanism of protein kinase CK2. The N-terminal segment is essential for constitutive activity of the catalytic subunit but not of the holoenzyme. J Biol Chem 277: 22509–22514, 2002
Lin WJ, Sheu GT, Traugh JA: Effects of autophosphorylation on casein kinase II activity: Evidence from mutations in the beta subunit. Biochemistry 33: 6998–7004, 1994
Jauch E, Melzig J, Brkulj M, Raabe T: In vivo functional analysis of Drosophila protein kinase casein kinase 2 (CK2) beta-subunit. Gene 298: 29–39, 2002
Karandikar UC, Trott RL, Yin J, Bishop CP, Bidwai AP: Drosophila CK2 regulates eye morphogenesis via phosphorylation of E(spl)M8. Mech Dev 121: 273–286, 2004
Willert K, Brink M, Wodarz A, Varmus H, Nusse R: Casein kinase 2 associates with and phosphorylates dishevelled. Embo J 16: 3089–30896, 1997
Niefind K, Guerra B, Pinna LA, Issinger OG, Schomburg D: Crystal structure of the catalytic subunit of protein kinase CK2 from Zea mays at 2.1 A resolution. Embo J 17: 2451–2462, 1998
Nawathean P, Rosbash M: The doubletime and CKII kinases collaborate to potentiate Drosophila PER transcriptional repressor activity. Mol Cell 13: 213–223, 2004
Konopka RJ, Smith RF, Orr D: Characterization of Andante, a new Drosophila clock mutant, and its interactions with other clock mutants. J Neurogenet 7: 103–114, 1991
Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T, Jackson FR: A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci 6: 251–257, 2003
Chaillot D, Declerck N, Niefind K, Schomburg D, Chardot T, Meunier JC: Mutation of recombinant catalytic subunit alpha of the protein kinase CK2 that affects catalytic efficiency and specificity. Protein Eng 13: 291–298, 2000
Glover CV: A filamentous form of Drosophila casein kinase II. J Biol Chem 261: 14349–14354, 1986
Valero E, De Bonis S, Filhol O, Wade RH, Langowski J, Chambaz EM, Cochet C: Quaternary structure of casein kinase 2. Characterization of multiple oligomeric states and relation with its catalytic activity. J Biol Chem 270: 8345–8352, 1995
Rekha N, Srinivasan N: Structural basis of regulation and substrate specificity of protein kinase CK2 deduced from the modeling of protein–protein interactions. BMC Struct Biol 3: 4, 2003
Yang Y, Cheng P, Liu Y: Regulation of the Neurospora circadian clock by casein kinase II. Genes Dev 16: 994–1006, 2002
Sugano S, Andronis C, Green RM, Wang ZY, Tobin EM: Protein kinase CK2 interacts with and phosphorylates the Arabidopsis circadian clock-associated 1 protein. Proc Natl Acad Sci U S A 95: 11020–11025, 1998
Sugano S, Andronis C, Ong MS, Green RM, Tobin EM: The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc Natl Acad Sci U S A 96: 12362–12366, 1999
Dunlap JC, Loros JJ: The neurospora circadian system. J Biol Rhythms 19: 414–424, 2004
Liu Y, Loros J, Dunlap JC: Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc Natl Acad Sci U S A 97: 234–239, 2000
Yang Y, Cheng P, Liu Y: Regulation of the Neurospora circadian clock by casein kinase II. Genes Dev 16: 994–1006, 2002
Yang Y, Cheng P, He Q, Wang L, Liu Y: Phosphorylation of FREQUENCY protein by casein kinase II is necessary for the function of the Neurospora circadian clock. Mol Cell Biol 23: 6221 – 6228, 2003
Salome PA, McClung CR: The Arabidopsis thaliana clock. J Biol Rhythms 19: 425–435, 2004
Daniel X, Sugano S, Tobin EM: CK2 phosphorylation of CCA1 is necessary for its circadian oscillator function in Arabidopsis. Proc Natl Acad Sci U S A 101: 3292–3297, 2004
Bode AM, Dong Z: Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4: 793–805, 2004
Keller DM, Zeng X, Wang Y, Zhang QH, Kapoor M, Shu H, Goodman R, Lozano G, Zhao Y, Lu H: A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell 7: 283–292, 2001
Hupp TR, Meek DW, Midgley CA, Lane DP: Regulation of the specific DNA binding function of p53. Cell 71: 875–886, 1992
Kato T, Jr., Delhase M, Hoffmann A, Karin M: CK2 Is a C-Terminal IkappaB Kinase Responsible for NF-kappaB Activation during the UV Response. Mol Cell 12: 829–839, 2003
Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, Sarno S, Meggio F, Pinna LA, Caldecott KW: The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell 117: 17–28, 2004
Krohn NM, Stemmer C, Fojan P, Grimm R, Grasser KD: Protein kinase CK2 phosphorylates the high mobility group domain protein SSRP1, inducing the recognition of UV-damaged DNA. J Biol Chem 278: 12710–12715, 2003
Pittendrigh CS: Biological clocks, the functions, ancient and modern, of biological oscillations. In: Science in the Sixties, Proceedings of the 1965 Cloudcroft Symposiurm. Air Force Office of Scientific Research, Arlington, VA, 1965, pp.~96–111
Pittendrigh CS: Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol 55: 16–54, 1993
Gehring W, Rosbash M: The coevolution of blue-light photoreception and circadian rhythms. J Mol Evol 57 Suppl 1: S286–S289, 2003
Cashmore AR, Jarillo JA, Wu YJ, Liu D: Cryptochromes: Blue light receptors for plants and animals. Science 284: 760–765, 1999.
Williams JA, Su HS, Bernards A, Field J, Sehgal A: A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science 293: 2251–2256, 2001
Coogan AN, Piggins HD: MAP kinases in the mammalian circadian system – key regulators of clock function. J Neurochem 90: 769–775, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allada, R., Meissner, RA. Casein kinase 2, circadian clocks, and the flight from mutagenic light. Mol Cell Biochem 274, 141–149 (2005). https://doi.org/10.1007/s11010-005-2943-1
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-2943-1