Abstract
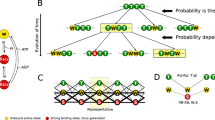
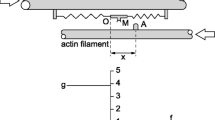
Two- and three-state cross-bridge models are considered and examined with respect to their ability to predict three distinct phases of the force transients that occur in response to step change in muscle fiber length. Particular attention is paid to satisfying the Le Châtelier–Brown Principle. This analysis shows that the two-state model can account for phases 1 and 2 of a force transient, but is barely adequate to account for phase 3 (delayed force) unless a stretch results in a sudden increase in the number of cross-bridges in the detached state. The three-state model \(({\mathbf{A \rightarrow B\rightarrow C\rightarrow A}})\) makes it possible to account for all three phases if we assume that the \({\mathbf{A\rightarrow B}}\) transition is fast (corresponding to phase 2), the \({\mathbf{B \rightarrow C}}\) transition is of intermediate speed (corresponding to phase 3), and the \({\mathbf{C \rightarrow A}}\) transition is slow; in such a scenario, states A and C can support or generate force (high force states) but state B cannot (detached, or low-force state). This model involves at least one ratchet mechanism. In this model, force can be generated by either of two transitions: \({\mathbf{B\rightarrow A}}\) or \({\mathbf{B\rightarrow C}}\). To determine which of these is the major force-generating step that consumes ATP and transduces energy, we examine the effects of ATP, ADP, and phosphate (Pi) on force transients. In doing so, we demonstrate that the fast transition (phase 2) is associated with the nucleotide-binding step, and that the intermediate-speed transition (phase 3) is associated with the Pi-release step. To account for all the effects of ligands, it is necessary to expand the three-state model into a six-state model that includes three ligand-bound states. The slowest phase of a force transient (phase 4) cannot be explained by any of the models described unless an additional mechanism is introduced. Here we suggest a role of series compliance to account for this phase, and propose a model that correlates the slowest step of the cross-bridge cycle (transition \({\mathbf{C\rightarrow A}}\)) to: phase 4 of step analysis, the rate constant k tr of the quick-release and restretch experiment, and the rate constant k act for force development time course following Ca2+ activation.















Similar content being viewed by others
References
Abbott RH (1973) An interpretation of the effect of fiber length and calcium on the mechanical properties of insect flight muscle. Cold Spring Harb Symp Quant Biol 37:647–654
Abbott RH, Steiger GJ (1977) Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J Physiol 266:13–42
Araujo A, Walker JW (1996) Phosphate release and force generation in cardiac myocytes investigated with caged phosphate and caged calcium. Biophys J 70:2316–2326
Bagni MA, Cecchi G, Colomo F and Poggesi C (1990) Tension and stiffness of frog muscle fibres at full filament overlap. J Muscle Res Cell Motil 11:371–377
Bagshaw CR, Eccleston JF, Eckstein F, Goody RS, Gutfreund H, Trentham DR (1974) The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem J 141:351–364
Barsotti RJ, Ferenczi MA (1988) Kinetics of ATP hydrolysis and tension production in skinned cardiac muscle of the guinea pig. J Biol Chem 263:16750–16756
Bershitsky SY, Tsaturyan AK (1992) Tension responses to joule temperature jump in skinned rabbit muscle fibres. J Physiol 447:425–448
Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85:3265–3269
Brenner B, Schoenberg M, Chalovich JM, Greene LE, Eisenberg E (1982) Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci USA 79:7288–7291
Burton K, Simmons RM, Sleep J (2006) Kinetics of force recovery following length changes in active skinned single fibres from rabbit psoas muscle. J Physiol 573(2):305–328
Campbell KB, Razumova MV, Kirkpatrick RD, Slinker BK (2001) Nonlinear myofilament regulatory processes affect frequency-dependent muscle fiber stiffness. Biophys J 81:2278–2296
Cooke R (2005) Milestone in physiology: the sliding filament model: 1972–2004. J Gen Physiol 123:643–656
Cooke R, Pate E (1985) The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J 48:789–798
Coupland ME, Puchert E, Ranatunga KW (2001) Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J Physiol 536:879–891
Dantzig J, Goldman Y, Millar NC, Lacktis J, Homsher E (1992). Reversal of the cross-bridge force-generating transition by the photogeneration of phosphate in rabbit psoas muscle fibers. J Physiol 451:247–278
Davis JS, Rodgers ME (1995) Force generation and temperature-jump and length-jump tension transients in muscle fibers. Biophys J 68:2032–2040
Davis JS, Satorius CL, Epstein ND (2002) Kinetic effects of myosin regulatory light chain phosphorylation on skeletal muscle contraction. Biophys J 83:359–370
Dickinson MH, Hyatt CJ, Lehmann F-O, Moore JR, Reedy MC, Simcox A, Tohtong R, Vigoreaux JO, Yamashita H, Maughan DW (1997) Phosphorylation-dependent power output of transgenic flies: an integrated study. Biophys J 73:3122–3134
Dobbie I, Linari M, Piazzesi G, Reconditi M, Koubassova N, Ferenczi M, Lombardi V, Irving M (1998) Elastic bending and active tilting of myosin heads during muscle contraction. Nature 396:383–387
Dominguez R, Freyzon Y, Trybus KM, Cohen C (1998) Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualisation of the pre-power stroke state. Cell 94:559–571
Fenn WO (1923) A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol 58:175–203
Finer JT, Simmons RM, Spudich JA (1994) Single myosin mechanics: piconewton forces and nanometre steps. Nature (Lond) 368:113–119
Ford LE, Huxley AF, Simmons RM (1977) Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol 269:441–515
Fortune NS, Geeves MA, Ranatunga KW (1991) Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proc Natl Acad Sci USA 88:7323–7327
Fujita H, Sasaki D, Ishiwata S, Kawai M (2002) Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys J 82:915–928
Galler S, Wang BG, Kawai M (2005) Elementary steps of the cross-bridge cycle in fast-twitch fiber types from rabbit skeletal muscles. Biophys J 89:3248–3260
Geeves MA, Holmes KC (1999) Structural mechanism of muscle contraction. Annu Rev Biochem 68:687–728
Geeves MA, Holmes KC (2005) The molecular mechanism of muscle contraction. Adv Protein Chem 71:161–193
Goldman YE, Hibberd MG, Trentham DR (1984) Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5’-triphosphate. J Physiol 354:577–604
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924
Guilford WH, Dupuis DE, Kennedy G, Wu J, Patlak JB, Warshaw DM (1997) Smooth muscle and skeletal muscle myosins produce similar unitary forces and displacements in the laser trap. Biophys J 72:1006–1021
Gutfreund H (1995) Kinetics for the life sciences. Receptors, transmitters and catalysts. Cambridge University Press
Heinl P, Kuhn HJ, Rüegg JC (1974) Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol 237:243–258
Herrmann C, Lionne C, Travers F, Barman T (1994) Correlation of ActoS1, myofibrillar, and muscle fiber ATPases. Biochemistry 33:4148–4154
Higuchi H, Yanagida T and Goldman YE (1995) Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J 69:1000–1010
Homsher W, Millar NC (1990) Caged compounds and striated muscle contraction. Annu Rev Physiol 52:875–896
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Huxley AF (1974) Muscular contraction. J Physiol 243:1–43
Huxley AF (1980) Reflections on muscle. Princeton University Press, Princeton
Huxley AF, Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Huxley HE, Stewart A, Sosa H, Irving T (1994). X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J 67:2411–2421
Ishijima A, Kojima H, Higuchi H, Harada Y, Funatsu T, Yanagida T (1996) Multiple- and single-molecule analysis of the actomyosin motor by nanometer-piconewton manipulation with a microneedle: unitary steps and force. Biophys J 70:383–400
Julian FJ, Sollins KR, Sollins MR (1974) A model for the transient and steady-state mechanical behavior of contracting muscle. Biophys J 14:546–562
Kawai M (1978) Head rotation or dissociation? A study of exponential rate processes in chemically skinned rabbit muscle fibers when MgATP concentration is changed. Biophys J 22:97–103
Kawai M (1986) The role of orthophosphate in crossbridge kinetics in chemically skinned rabbit psoas fibers as detected with sinusoidal and step length alterations. J Muscle Res Cell Motil 7:421–434
Kawai M (2003) What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres? J Muscle Res Cell Motil 24:127–138
Kawai M, Brandt PW (1980) Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil 1:279–303
Kawai M, Halvorson HR (1989) Role of MgATP and MgADP in the crossbridge kinetics in chemically skinned rabbit psoas fibers. Study of a fast exponential process (C). Biophys J 55:595–603
Kawai M, Halvorson HR (1991) Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned rabbit psoas muscle. Biophys J 59:329–342
Kawai M, Schachat FH (1984) Differences in the transient response to fast and slow skeletal muscle fibers: correlations between complex modulus and myosin light chains. Biophys J 45:1145–1151
Kawai M, Zhao Y (1993) Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibers. Biophys J 65:638–651
Kawai M, Saeki Y, Zhao Y (1993) Cross-bridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res 73:35–50
Kirkwood JG, Oppenheim I (1961) Chemical thermodynamics. McGraw-Hill Book Co. Inc., New York
Kitamura K, Tokunaga M, Iwane AH, Yanagida T (1999) A single myosin head moves along an actin filament with regular steps of 5.3 nanometres. Nature 397(6715):129–134
Kojima H, Ishijima A, Yanagida T (1994). Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation. Proc Natl Acad Sci USA 91:12962–12966
Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci 100:13716–13721
Lu X, Bryant MK, Bryan KE, Rubenstein PA, Kawai M (2005) Role of the N-terminal negative charge of actin in cross-bridge kinetics and force generation in reconstituted bovine myocardium. J Physiol 564(1):65–82
Lymn RW, Taylor EW (1971) Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10:4617–4624
Marcussen BL, Kawai M (1990) Role of MgATP and inorganic phosphate ions in crossbridge kinetics in insect (Lethocerus colossicus) flight muscle. In: Sperelakis N, Wood JD (eds) Frontiers in smooth muscle research. Wiley-Liss, New York, pp 805–813
Martin H, Barsotti RJ (1994) Relaxation from rigor of skinned trabeculae of the guinea pig induced by laser photolysis of caged ATP. Biophys J 66:1115–1128
Meyer RA, Brown TR, Kushmerick MJ (1985) Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol 248:C279–C287
Mijailovich SM, Fredberg JJ, Butler JP (1996) On the theory of muscle contraction: filament extensibility and the development of isometric force and stiffness. Biophys J 71:1475–1484
Millar NC, Geeves MA (1988) Protein fluorescence changes associated with ATP and adenosine 5’-[γ-thio]triphosphate binding to skeletal muscle myosin subfragment 1 and actomyosin subfragment 1. Biochem J 249:735–743
Millar NC, Homsher E (1992) Kinetics of force generation and phosphate release in skinned rabbit soleus muscle fibers. Am J Physiol 252(Cell Physiol 31):C1239–C1245
Miyata H, Yoshikawa H, Hakozaki H, Suzuki N, Furuno T, Ikegami A, Kinosita K Jr, Nishizaka T, Ishiwata S (1995) Mechanical measurements of single actomyosin motor force. Biophys J 68:286S–290S
Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DCS (1995) Movement and force produced by a single myosin head. Nature (Lond) 378:209–212
Murase M, Tanaka H, Nishiyama K, Shimizu H (1986) A three-state model for oscillation in muscle: sinusoidal analysis. J Muscle Res Cell Motil 7:2–10
Palmer BM, Suzuki T, Wang Y, Barnes WD, Miller MS, Maughan DW (2007) Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J 93:760–769
Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V (2007) Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131:784–795
Piroddi N, Tesi C, Pellegrino MA, Tobacman LS, Homsher E, Poggesi C (2003) Contractile effects of the exchange of cardiac troponin for fast skeletal troponin in rabbit psoas single myofibrils. J Physiol 552.3:917–931
Preston LC, Lipscomb S, Robinson P, Mogensen J, McKenna WJ, Watkins H, Ashley CC, Redwood CS (2007) Functional effects of the DCM mutant Gly159Asp troponin C in skinned muscle fibres. Pflugers Arch 453:771–776
Pringle JWS (1978) Stretch activation of muscle: function and mechanism. Proc R Soc Lond B 201:107–130
Ranatunga KW (1999) Effects of inorganic phosphate on endothermic force generation in muscle. Proc Biol Sci 266:1381–1385
Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM (1993) Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261:50–58
Regnier M, Morris C, Homsher E (1995) Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am J Physiol 269(6 Pt 1):C1532–C1539
Rosenfeld SS, Xing J, Whitaker M, Cheung HC, Brown F, Wells A, Milligan RA, Sweeney HL (2001) Kinetic and spectroscopic evidence for three actomyosin: ADP states in smooth muscle. J Biol Chem 275:25418–25426
Saeki Y, Kawai M, Zhao Y (1991) Comparison of crossbridge dynamics between intact and skinned myocardium from ferret right ventricles. Circ Res 68:772–781
Saeki Y, Sagawa K, Suga H (1978) Dynamic stiffness of cat heart muscle in Ba2+-induced contracture. Circ Res 42:324–333
Sleep JA, Hutton RL (1980) Exchange between inorganic phosphate and adenosine 5’-triphosphate in the medium by actomyosin subfragment 1. Biochemistry 19:1276–1283
Smith DA (1998) A strain-dependent ratchet model for [phosphate]- and [ATP]-dependent muscle contraction. J Muscle Res Cell Motil 19:189–211
Smith DA, Geeves MA (1995) Strain-dependent cross-bridge cycle for muscle. Biophys J 69:524–537
Spirito P, Seidman CE, McKenna WJ, Maron BJ (1997) The management of hypertrophic cardiomyopathy. N Engl J Med 336:775–785
Squire JM, Luther PK, Knupp C (2003) Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol 331:713–724
Stehle R, Krüger M, Scherer P, Brixius K, Schwinger RH, Pfitzer G (2002) Isometric force kinetics upon rapid activation and relaxation of mouse, guinea pig and human heart muscle studied on the subcellular myofibrillar level. Basic Res Cardiol 97(Suppl 1):I127–I135
Steiger GJ, Abbott RH (1981) Biochemical interpretation of tension transients produced by a four-state mechanical model. J Muscle Res Cell Motil 2:245–260
Stein LA, Greene LE, Chock PB, Eisenberg E (1985) Rate-limiting step in the actomyosin adenosinetriphosphatase cycle: studies with myosin subfragment 1 cross-linked to actin. Biochemistry 24:1357–1363
Swank DM, Vishnudas VK, Maughan DW (2006) An exceptionally fast actomyosin reaction powers insect flight muscle. Proc Natl Acad Sci USA 103:17543–17547
Takagi Y, Shuman H, Goldman YE (2004) Coupling between phosphate release and force generation in muscle actomyosin. Philos Trans R Soc Lond B Biol Sci 359:1913–1920
Tanner BC, Daniel TL, Regnier M (2007) Sarcomere lattice geometry influences cooperative myosin binding in muscle. PLoS Comput Biol 3:1195–1211
Tawada K, Kawai M (1990) Covalent cross-linking of single muscle fibers from rabbit psoas increases oscillatory power. Biophys J 57:643–647
Taylor EW (1979) Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem 6:103–164
Tesi C, Colomo F, Nencini S, Piroddi N, Poggesi C (2000) The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys J 78:3081–3092
Tesi C, Colomo F, Piroddi N, Poggesi C (2002) Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J Physiol 541(1):189–199
Thirlwell H, Sleep JA, Ferenczi MA (1995) Inhibition of unloaded shortening velocity in permeabilized muscle fibres by caged ATP compounds. J Muscle Res Cell Motil 16:131–137
Thorson J, White DC (1969) Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J 9:360–390
Thorson J, White DC (1983) Role of cross-bridge distortion in the small-signal mechanical dynamics of insect and rabbit striated muscle. J Physiol 343:59–84
Tikunov BA, Sweeney HL, Rome LC (2000) Quantitative electrophoretic analysis of myosin heavy chains in single muscle fibers. J Appl Physiol 90:1927–1935
Vale RD, Oosawa F (1990) Protein motors and Maxwell’s demons: does mechanochemical transduction involve a thermal ratchet? Adv Biophys 26:97–134
Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y and Amemiya Y (1994) X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J 67: 2422–2435
Walker JW, Lu Z, Moss RL (1992) Effects of Ca2+ on the kinetics of phosphate release in skeletal muscle. J Biol Chem 267:2459–2466
Wang G, Kawai M (1996) Effects of MgATP and MgADP on the cross-bridge kinetics of rabbit soleus slow-twitch muscle fibers. Biophys J 71:1450–1461
Wang G, Kawai M (1997) Force generation and phosphate release steps in skinned rabbit soleus slow-twitch muscle fibers. Biophys J 73:878–894
Wang G, Ding W, Kawai M (1999) Does thin filament compliance diminish the cross-bridge kinetics? A study in rabbit psoas fibers. Biophys J 76:978–984
Wannenburg T, Heijne GH, Geerdink JH, Van Den Dool HW, Janssen PM, De Tombe PP (2000) Cross-bridge kinetics in rat myocardium: effect of sarcomere length and calcium activation. Am J Physiol 279: H779–H790
Webb MR, Hibberd MG, Goldman YE, Trentham DR (1986) Oxygen exchange between Pi in the medium and water during ATP hydrolysis mediated by skinned fibers from rabbit skeletal muscle: evidence for Pi binding to a force-generating state. J Biol Chem 261:15557–15564
White DC (1983) The elasticity of relaxed insect fibrillar flight muscle. J Physiol 343:31–57
White HD, Taylor EW (1976) Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry 15:5818–5826
Woledge RC, Curtin NA, Homsher E (1985) Energetic aspects of muscle contraction (Monographs of the Physiological Society, No. 41). Academic Press, New York
Xu S, Offer G, Gu J, White HD, Yu LC (2003) Temperature and ligand dependence of conformation and helical order in myosin filaments. Biochemistry 42:390–401
Yates LD, Greaser ML (1983) Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol 168:123–141
Yount RG, Lawson D, Rayment I (1995) Is myosin a “back door” enzyme? Biophys J 68:44S–47S
Zhao Y, Kawai M (1993) The effect of the lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J 64:197–210
Zhao Y, Kawai M (1994) Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J 67:1655–1668
Zhao Y, Kawai M (1996) Inotropic agent EMD 53998 weakens nucleotide and phosphate binding to cross bridges in porcine myocardium. Am J Physiol 271(Heart Circ Physiol 40):H1394–H1406
Zhao Y, Swamy PMG, Humphries KA, Kawai M (1996) The effect of partial extraction of troponin C on the elementary steps of the cross-bridge cycle in rabbit psoas fibers. Biophys J 71:2759–2773
Acknowledgements
The authors are indebted to Dr. David W. Maughan of the University of Vermont (USA) for his critical reading of the manuscript and his constructive comments and useful suggestions. The authors are also grateful to Dr. Christine Blaumueller for her critical reading of the manuscript and creative suggestions. This work was supported by a grant from NIH HL70041 to MK. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of the awarding organization.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
The solution of the differential equation (A1) can be found in the following way.
Now we substitute Y(t) with Z(t) so that
is satisfied. From Eq. A2,
By substituting Eqs. A2 and A3 to Eq. A1, we arrive at
By integrating Eq. A4, we get
where Y 0 is the integration constant. From Eqs. A2 and A5, we get
Therefore, Eq. A1 has an exponential process with the rate constant λ, amplitude Y 0, and the steady state value h/λ (Eq. A6). Y 0 is determined by the initial conditions.
Appendix 2
In Scheme 3, the following differential equations can be set up.
Eqs. A7–A9 can be written in matrix form.
Note the similarity of Eqs. A1 and A10. Note also that |H| = det(H) = 0, which is consistent with Eq. A13. The eigen values (λ) of matrix H can be found by determining the roots of Eq. A14.
where I is the identity matrix. From Eq. A14,
Equation A15 has three roots λ1, λ2, and λ3.
With Eqs. A17 and A18, Eq. A10 can be solved to result in:
The elements U i2 and U i3 (i = 1, 2, 3) are 2 eigen vectors (column vectors) of matrix H (Eq. A12) corresponding to λ2 and λ3, respectively, and they are time-independent variables. Their size (length) is determined by the initial conditions (2 independent variables). A 1, B 1 and C 1 are the steady-state concentrations (see below). The correctness of Eq. A19 can be examined by substituting it into Eq. A10 keeping in mind that:
From Eqs. A19 and A20, the individual solutions are:
Therefore, according to Eq. 34, a force transient has two exponential processes that correspond to phases 2 and 3, with the rate constants λ2 and λ3, respectively.
If R < 0, then λ2, λ3, U i2 and U i3 are complex numbers, and λ3 = λ2* (Eqs. A17 and A18), and U i3 = U i2*, where * indicates the complex conjugate. In this case, Eq. A19 can be rearranged to result:
where \({\Re}(U_{ij})\)refers to the real, and \({\Im}(U_{ij})\) to the imaginary part of the complex number U ij . Equation A24 shows that this system has a damped oscillation. However, in literature dealing with muscle fibers, it is rare to find force transients with a damped oscillation in response to a sudden change in an experimental condition. If a force transient should oscillate, a resonance of the force transducer should be suspected before a conclusion is drawn. The fact that an oscillation is absent implies that the intrinsic rate constants among the three steps in Scheme 3 differ significantly.
Steady state
By setting dA/dt = 0 and dB/dt = 0 in Eqs. A7 and A8, and with the constraints of Eq. A13, we can solve the steady-state concentrations of A 1, B 1 and C 1. These three values constitute an eigen vector (column vector) which belongs to the eigen value λ1. M is defined in Eq. A16.
The turnover (ATP hydrolysis) rate is:
A similar analysis based on Scheme 8 is found in the Appendix of Kawai (2003).
Rights and permissions
About this article
Cite this article
Kawai, M., Halvorson, H.R. Force transients and minimum cross-bridge models in muscular contraction. J Muscle Res Cell Motil 28, 371–395 (2007). https://doi.org/10.1007/s10974-008-9131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-008-9131-3