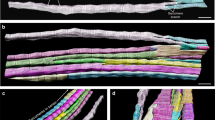
Abstract
Building a myofibril from its component proteins requires the interactions of many different proteins in a process whose details are not understood. Several models have been proposed to provide a framework for understanding the increasing data on new myofibrillar proteins and their localizations during muscle development. In this article we discuss four current models that seek to explain how the assembly occurs in vertebrate cross-striated muscles. The models hypothesize: (a) stress fiber-like structures as templates for the assembly of myofibrils, (b) assembly in which the actin filaments and Z-bands form subunits independently from A-band subunits, with the two subsequently joined together to form a myofibril, (c) premyofibrils as precursors of myofibrils, or (d) assembly occurring without any intermediary structures. The premyofibril model, proposed by the authors, is discussed in more detail as it could explain myofibrillogenesis under a variety of different conditions: in ovo, in explants, and in tissue culture studies on cardiac and skeletal muscles.
Similar content being viewed by others
References
Almenar-Queralt A, Gregorio CC, Fowler VM, (1999) Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils J Cell Sci 112:1111–1123
Antin PB, Forry-Schaudies S, Friedman TM, Tapscott SJ, Holtzer H, (1981) Taxol induces postmitotic myoblasts to assemble interdigitating microtubule-myosin arrays that exclude actin filaments J Cell Biol 90: 300–308
Ayoob JC, Shaner NC, Sanger JM, Sanger JW, (2001) Expression of Green or Red Fluorescent Protein (GFP or DsRed) linked proteins in Non-muscle and Muscle Cells Mol Biotechnol 17: 65–71
Buxton DB, Golomb E, Adelstein RS, (2003) Induction of nonmuscle myosin heavy chain II-C by butyrate in RAW 264.7 mouse macrophages J Cell Biol 278: 15449–15455
Chowrashi P, Mittal B, Sanger JM, Sanger JW, (2002) Amorphin is phosphorylase; phosphorylase is an alpha-actinin-binding protein Cell Motil Cytoskeleton 53: 125–135
Clark KA, McElhinny AS, Beckerle MC, Gregorio CC, (2002) Striated muscle cytoarchitecture: an intricate web of form and function Ann Rev Cell Dev Biol 18: 637–706
Claycomb WC, Palazzo MC, (1980) Culture of the terminally differentiated adult cardiac muscle cell: a light and scanning electron microscope study Dev Biol 80: 466–482
Conrad AH, Jaffredo T, Conrad GW, (1995) Differential localization of cytoplasmic myosin II isoforms A and B in avian interphase and dividing embryonic and immortalized cardiomyocytes and other cell types in vitro Cell Motil Cytoskeleton 31: 93–112
Costa ML, Escaleira RC, Rodrigues VB, Manasfi M, Mermelstein CS, (2002) Some distinctive features of zebrafish myogenesis based on unexpected distributions of the muscle cytoskeletal proteins actin, myosin, desmin, alpha-actinin, troponin and titin Mech Dev 116: 95–104
Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW, (1997) Myofibrillogenesis visualized in living embryonic cardiomyocytes Proc Natl Acad Sci USA 94:9493–9498
Dabiri GA, Turnacioglu KK, Ayoob JC, Sanger JM, Sanger JW, (1999a) Transfections of primary muscle cell cultures with plasmids coding for GFP linked to full-length and truncated muscle proteins Method Cell Biol 58:239–260
Dabiri GA, Ayoob JP, Turnacioglu KK, Sanger JM, Sanger JW, (1999b) Use of Green Fluorescent Proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells Method Enzymol 302:171–186
Dlugosz AA, Antin PB, Nachmias VT, Holtzer H, (1984) The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes J Cell Biol 99: 2268–2278
Du A, Sanger JM, Linask KK, Sanger JW, (2003a) Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm Dev Biol 57: 382–394
Du A., Sanger JM, Sanger JW, (2003b) Cardiac myofibrillogenesis follows similar pathways in ovo, in explants, and in tissue culture Mol Biol Cell 14: 423a
Ehler E, Rothen BM, Haemmerle SP, Komiyama M, Perriard JC, (1999) Myofibrillogenesis in the developing chicken heart: assembly of Z-disk, M-line and the thick filaments J Cell Sci 112: 1529–1539
Engel AG, Banker BQ, (2004) Ultrastructural changes in diseased muscle. In: Engel AG, Franzini-Armstrong C, (eds.) Myology. (3rd Edition), McGraw-Hill, New York, 749–887
Ervasti JM, (2003) Costameres: the Achilles’ heel of Herculean muscle J Biol Chem 278: 13591–13594
Fallon JR, Nachmias VT, 1980. Localization of cytoplasmic and skeletal myosins in developing muscle cells by double-label immunofluorescence J Cell Biol 87:237–247
Faulkner G, Lanfranchi G, Valle G, (2001). Telethonin and other new proteins of the Z-disc of skeletal muscle IUBMB Life 51: 275–282
Feramisco JR, (1979) Microinjection of fluorescently labeled alpha-actinin into living fibroblasts Proc Natl Acad Sci USA 76: 3967–39671
Ferrari MB, Ribbeck K, Hagler DJ, Spitzer NC, (1998) A calcium cascade essential for myosin thick filament assembly in Xenopus myocytes J Cell Biol 141:1349–1356
Fischman DA, (1967) An electron microscope study of myofibril formation in embryonic chick skeletal muscle J Cell Biol 32: 557–575
Goldstein MA, Cartwright J, (1982) Microtubules in adult mammalian muscle. In: Dowben RM, Shay JW, (eds.) Cell and Muscle Motility Plenum Press New York, 85–92
Golomb E, Ma X., Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS, (2004) Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family J Biol Chem 279: 2800–2808
Golson ML, Sanger JM, Sanger JW, (2004) Inhibitors arrest myofibrillogenesis in skeletal muscle cells at early stages of assembly Cell Motil Cytoskeleton 59:1–16
Gregorio CC, Antin PB, (2000) To the heart of myofibril assembly Trends Cell Biol 10: 355–362
Gundersen GG, Khawaja S, Bulinski JC, (1989) Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation J Cell Biol 109: 2275–2288
Holtzer H, Hijikata T, Lin ZX, Zhang ZQ, Holtzer S, Protasi F, Franzini-Armstrong C, Sweeney HL, (1997) Independent assembly of 1.6 micron long bipolar MHC filaments and I-Z-I bodies Cell Struc Funct 22:83–93
Imanaka-Yoshida K, Danowski BA, Sanger JM, Sanger JW, (1996) Living adult rat cardiomyocytes in culture: evidence for dissociation of costameric distribution of vinculin from costameric distributions of attachments Cell Motil Cytoskeleton 33: 263–275
Kaneko H, Okamoto M, Goshima K, (1984) Structural change of myofibrils during mitosis of newt embryonic myocardial cells in culture Exp Cell Res 153: 483–498
Kelly DE, (1969) Myofibrillogenesis and Z-band differentiation Anat Rec 163: 403–426
Kelly AM, Chacko S, (1976) Myofibril organisation and mitosis in cultured cardiac muscle cells Dev Biol 48: 421–430
Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR, (2002) The MLP family of cytoskeletal Z disc proteins and dilated cardiomyopathy: a stress pathway model for heart failure progression Cold Spring Harb Symp Quant Biol 67: 399–408
Langanger G, Moeremans M, Daneels G, Sobieszek A, De Brabander M, De Mey J, (1986) The molecular organization of myosin in stress fibers of cultured cells J Cell Biol 102: 200–209
LoRusso SM, Rhee D, Sanger JM, Sanger JW, (1997) Premyofibrils in spreading adult cardiomyoctes in tissue culture: evidence for re-expression of the embryonic program in adult cells Cell Motil Cytoskeleton 37:183–198
Lu MH, DiLullo C, Schultheiss T, Holtzer S, Murray JM, Choi J, Fischman DA, Holtzer H, (1992) The vinculin/sarcomeric-alpha-actinin/alpha-actin nexus in cultured cardiac myocytes J Cell Biol 117: 1007–1022
Mangan ME, Olmsted JB, (1996) A muscle-specific variant of microtubule-associated protein 4 (MAP4) is required in myogenesis Development 122: 771–781
Maruyama KM, Kuroda M, Nonomura Y, (1985) Association of chicken pectoralis muscle phosphorylase with the Z-line and the M-line of myofibrils: comparison with ‘amorphin’, the amorphous component of the Z-line Biochim Biophys Acta 829: 229–237
McKenna NM, Meigs JB, Wang YL, (1985) Exchangeability of alpha-actinin in living cardiac fibroblasts and muscle cells J Cell Biol 101: 2223–2232
Mitchell PO, Pavlath GK, (2002) Multiple roles of calcineurin in skeletal muscle growth Clin Orthop 403S: S197–S202
Mittal B, Sanger JM, Sanger JW, (1987) Binding and distribution of filamin in permeabilized and living non-muscle and muscle cells Cell Motil Cytoskeleton 8:345–359
Moncman CL, Wang K, (1996) Assembly of nebulin into the sarcomeres of avian skeletal muscle Cell Motil Cytoskeleton 34: 167–184
Peng HB, Wolosewick JJ, Cheng PC, (1981) The development of myofibrils in cultured muscle cells: a whole-mount and thin-section electron microscopic study Dev Biol 88: 121–136
Rhee D, Sanger JM, Sanger JW, 1994. The premyofibril: evidence for its role in myofibrillogenesis Cell Motil Cytoskeleton 28:1–24
Rudy DE, Yatskievych TA, Antin PB, Gregorio CC, (2001) Assembly of thick, and thin, titin filaments in chick precardiac explants Dev Dyn 221: 61–71
Saitoh O, Arai T, Obinata T, (1988) Distribution of microtubules and other cytoskeletal filaments during myotube elongation as revealed by fluorescence microscopy Cell Tissue Res 252: 263–273
Sanger JM, Sanger JW, (1980) Banding and polarity of actin filaments in interphase and cleaving cells J Cell Biol 86:568–575
Sanger JM, Sanger JW, (2000) Assembly of cytoskeletal proteins into cleavage furrows of tissue culture cells Microsc Res Techniq 49: 190–201
Sanger JW, Sanger JM, (2001a) Fishing out proteins that bind to titin J Cell Biol 154:21–24
Sanger JW, Sanger JM, (2001b) Green fluorescent proteins improve myofibril research Biophoton Int 8: 44–46
Sanger JW, Sanger JM, (2002) Myofibrillogenesis in cardiac muscle cells. In: Dube D, (ed.) Myofibrillogenesis. Springer Verlag, New York, 3–20
Sanger JW, Mittal B, Sanger JM, (1984) Formation of myofibrils in spreading chick cardiac myocytes Cell Motil 4:405–416
Sanger JM, Mittal B, Pochapin MB, Sanger JW, (1986a) Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin J Cell Biol 102:2053–2066
Sanger JM, Mittal B, Pochapin MB and Sanger JW (1986b) Observations of microfilament bundles in living cells microinjected with fluorescently labeled contractile proteins. J Cell Sci Suppl 5: 17–44
Sanger JW, Ayoob JC, Chowrashi P, Zurawski. D, Sanger JM, (2000) Assembly of myofibrils in cardiac muscle cells Advan Exper Med Biol 481: 89–102
Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman N, Sanger JM, (2002) Myofibrillogenesis in skeletal muscle cells Clin Ortho 403S: S153–S162
Sanger JW, Sanger JM, Franzini-Armstrong C, (2004) Assembly of the skeletal muscle cell. In: Engel AG, Franzini-Armstrong C, (eds.) Myology. (3rd Edition), McGraw-Hill New York, 45–65
Schaub MC, Hefti MA, Harder BA, Eppenberger HM, (1997) Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes J Mol Med 75: 901–920
Siebrands CC, Sanger JM, Sanger JW, (2004) Myofibrillogenesis in skeletal muscle cells in the presence of taxol Cell Motil Cytoskeleton 58: 39–52
Toyama Y, Forry-Schaudies S, Hoffman B, Holtzer H, (1982) Effects of taxol and colcemid on myofibrillogenesis Proc Natl Acad Sci USA 79: 6556–6560
Tskhovrebova L, Trinick J, (2004) Titin: properties and family relationships Nat Rev Mol Cell Biol 4: 679–689
Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS, (1997) Nonmuscle myosin II-B is required for normal development of the mouse heart Proc Natl Acad Sci USA 94: 12407–12412
Turnacioglu KK, Mittal B, Sanger JM, Sanger JW, (1996) Partial characterization of zeugmatin indicates that is part of the Z-band region of titin Cell Motil Cytoskeleton 34:108–121
Wang J, Shaner NC, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW, (2005a) Dynamics of Z-band based proteins in developing skeletal muscle cells Cell Motil Cytoskeleton 61: 34–48
Wang J, Sanger JM and Sanger JW, (2005b) Differential effects of latrunculin-A on myofibrils in cultures of skeletal muscle cells: insights into mechanisms of myofibrillogenesis. Cell Motil Cytoskeleton 62: 35–47
Warren RH, (1968) The effect of colchicine on myogenesis in vivo in Rana pipiens and Rhodnius prolixus (Hemiptera) J Cell Biol 63: 550–566
Warren RH, (1974) Microtubular organization in elongating myogenic cells J Cell Biol 39: 544–555
Zhukarev V, Sanger JW, Sanger JM, Goldman Y, Shuman H, (1997) Steady state fluorescence polarization analysis of rhodamine phalloidin binding to muscle Cell Motil Cytoskeleton 37: 363–377
Acknowledgements
The authors are indebted to Ms. Victoria McManus for her comments on this manuscript. This work was supported by grants from AHA (JMS), MDA (JWS, JMS), and the National Institutes of Health (JWS, JMS); AHA fellowship (AD).
Author information
Authors and Affiliations
Corresponding author
Additional information
In memoriam: This paper is dedicated to the memory of Professor Koscak Maruyama, a noted contributor in the field of muscle biochemistry.
Rights and permissions
About this article
Cite this article
Sanger, J.W., Kang, S., Siebrands, C.C. et al. How to build a myofibril. J Muscle Res Cell Motil 26, 343–354 (2005). https://doi.org/10.1007/s10974-005-9016-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-005-9016-7