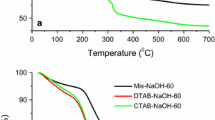
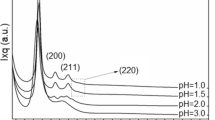
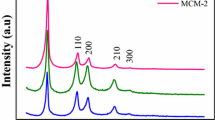
Abstract
A 24 factorial experiment program was carried out to study the main and interaction effects of four factors (mixture CTMABr/SiO2, H2O/SiO2, and EthAc/SiO2, and reaction time) on pore ordering, hexagonal unit cell parameter a0, and morphology of MCM-41. The MCM-41 was synthesized from a sodium silicate solution using cetyltrimethylammonium bromide (CTMABr) surfactant and ethyl acetate (EthAc) pH modifier. None of the factors acted independently to determine pore ordering, in contrast to earlier limited literature data, which suggested a higher CTMABr/SiO2 disturbs the assembly of the MCM-41 structure. However, there is no contradiction between these results considering that the poorly ordered product was obtained previously from a reaction mixture with the higher EthAc/SiO2 and lower H2O/SiO2, which are shown to hinder pore ordering. A combination of these factors, resulting in a higher concentration of acetic acid (hydrolysis of EthAc), and thus, in a lower mixture alkalinity, implies that the pH affects pore ordering in MCM-41. This is consistent with extensive literature data on this mesoporous material. A small (up to ∼5%) variation of a0 due to the reaction composition and time variation was insignificant compared to the reported doubling of a0 caused by the effects of varying the surfactant alkyl chain length, addition of swelling organic compounds, or hydrothermal restructuring. Particle morphology (hexagonal platelets, gyroids, and crescent-like or worm-shaped particles) depended on the combination of mixture CTMABr/SiO2, H2O/SiO2, and EthAc/SiO2. This is consistent with the literature evidence that morphogenesis of hexagonally ordered silica is a complex phenomenon involving a variety of reaction variables.
Similar content being viewed by others
References
J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T-W. Chu, D.H. Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins, and J.L. Schlenker, J. Am. Chem. Soc. 114, 10834 (1992).
A. Taguchi and F. Schüth, Micropor. Mesopor. Mater. 77, 1 (2005).
A. Corma, Chem. Rev. 97, 2373 (1997).
X.S. Zhao, G.Q. Lu, and G.J. Millar, Ind. Eng. Chem. Res. 35, 2075 (1996).
J.S. Beck and J.C. Vartuli, Curr. Opin. Solid State Mater. Sci. 1, 76 (1996).
A. Firouzi, D. Kumar, L.M. Bull, T. Besier, P. Sieger, Q. Huo, S.A. Walker, J.A. Zasadzinski, C. Glinka, J. Nicol, D. Margolese, G.D. Stucky, and B.F. Chmelka, Science 267, 1138 (1995).
A. Firouzi, F. Atef, A.G. Oertli, G.D. Stucky, and B.F. Chmelka, J. Am. Chem. Soc. 119, 3596 (1997).
G.J. de A.A. Soler-Illia, C. Sanchez, B. Lebeau, and J. Patarin, Chem. Rev. 102, 4093 (2002).
J. Frasch, B. Lebeau, M. Soulard, J. Patarin, and R. Zana, Langmuir 16, 9049 (2000).
H.B.S. Chan, P.M. Budd, and T. deV. Naylor, J. Mater. Chem. 11, 951 (2001).
D. Zhao, J. Sun, Q. Li, and G.D. Stucky, Chem. Mater. 12, 275 (2000).
G.A. Ozin, Can. J. Chem. 77, 2001 (1999).
P. Selvam, S.K. Bhatia, and C.G. Sonwane, Ind. Eng. Chem. Res. 40, 3237 (2001).
S. Biz and M.L. Occelli, Catal. Rev. – Sci. Eng. 40, 329 (1998).
R.G. Petersen, Design and Analysis of Experiments (Marcel Dekker, New York, 1985).
D.C. Montgomery, Design and Analysis of Experiments (Wiley, New York, 1976).
P.T. Tanev and T.J. Pinnavaia, Chem. Mater. 8, 2068 (1996).
G. Ø ye, J. Sjöblom, and M. Stöcker, Micropor. Mesopor. Mater. 34, 291 (2000).
R. Ryoo and J.M. Kim, J. Chem. Soc., Chem. Commun. 711 (1995).
H.-P. Lin, S. Cheng, and C.-Y. Mou, Micropor. Mater. 10, 111 (1997).
A. Monnier, F. Schüth, Q. Huo, D. Kumar, D. Margolese, R.X. Maxwell, G.D. Stucky, M. Krishnamurty, P. Petroff, A. Firouzi, M. Janicke, and B.F. Chmelka, Science 261, 1299 (1993).
K.J. Edler and J.W. White, Chem. Mater. 9, 1226 (1997).
G. Schulz-Ekloff, J. Rathouský, and A. Zukal, Int. J. Inorg. Mater. 1, 97 (1999).
Q. Cai, W.-Y. Lin, F.-S. Xiao, W.-Q. Pang, X.-H. Chen, and B.-S. Zou, Micropor. Mesopor. Mater. 32, 1 (1999).
C.-F. Cheng, D.H. Park, and J. Klinowski, J. Chem. Soc., Faraday Trans. 93, 193 (1997).
C.-F. Cheng, W. Zhou, D.H. Park, J. Klinowski, M. Hargreaves, and L.F. Gladden, J. Chem. Soc., Faraday Trans. 93, 359 (1997).
Z. Luan, C.-F. Cheng, W. Zhou, and J. Klinowski, J. Phys. Chem. 99, 1018 (1995).
G. Schulz-Ekloff, J. Rathouský, and A. Zukal, Micropor. Mesopor. Mater. 27, 273 (1999).
Y. Rohlfing, D. Wöhrle, M. Wark, G. Schulz-Ekloff, J. Rathouský, and A. Zukal, in Zeolites and Mesoporous Materials at the Dawn of the 21st Century, edited by A. Sayari, F. DiRenzo, F. Fajula, and J. Vedrine, Studies in Surface Science and Catalysis (Elsevier, Amsterdam, 2000), Vol. 129.
J. Rathouský, G. Schulz-Ekloff, J. Had, and A. Zukal, Phys. Chem. Chem. Phys. 1, 3053 (1999).
D. Khushalani, A. Kuperman, G.A. Ozin, K. Tanaka, J. Garcés, M.M. Olken, and N. Coombs, Adv. Mater. 7, 842 (1995).
R. Mokaya, Micropor. Mesopor. Mater. 44–45, 119 (2001).
R. Ryoo and S. Jun, J. Phys. Chem. B 101, 317 (1997).
Q. Huo, D.I. Margolese, and G.D. Stucky, Chem. Mater. 8, 1147 (1996).
U. Ciesla and F. Schüth, Micropor. Mesopor. Mater. 27, 131 (1999).
G. Engelhardt and D. Michel, High-Resolution Solid-State NMR of Silicates and Zeolites (Wiley, New York, 1987).
R.K. Iler, The Chemistry of Silica (Wiley, New York, 1979).
J.C. Vartuli, K.D. Schmitt, C.T. Kresge, W.J. Roth, M.E. Leonowicz, S.B. McCullen, S.D. Hellring, J.S. Beck, J.L. Schlenker, D.H. Olson, and E.W. Sheppard, Chem. Mater. 6, 2317 (1994).
M.T. Anderson, J.E. Martin, J.G. Odinek, and P.P. Newcomer, Chem. Mater. 10, 311 (1998).
F. Di Renzo, D. Desplantier, A. Galarneau, and F. Fajula, Catal. Today 66, 75 (2001).
T.R. Pauly, V. Petkov, Y. Liu, S.J.L. Billinge, and T.J. Pinnavaia, J. Am. Chem. Soc. 124, 97 (2002).
C-Y. Chen, H-X. Li, and M.E. Davis, Micropor. Mater. 2, 17 (1993).
N. Coustel, F. Di Renzo, and F. Fajula, J. Chem. Soc., Chem. Commun. 967 (1994).
N. Ulagappan and C.N.R. Rao, Chem. Commun. 2759 (1996).
A. Sayari, Y. Yang, M. Kruk, and M. Jaroniec, J. Phys. Chem. B 103, 3651 (1999).
A. Corma, Q. Kan, M.T. Navarro, J. Pérez-Pariente, and F. Rey, Chem. Mater. 9, 2123 (1997).
M. Kruk, M. Jaroniec, and A. Sayari, J. Phys. Chem. B 103, 4590 (1999).
H. Yang, N. Coombs, and G.A. Ozin, Nature 386, 692 (1997).
C.-F. Cheng, H. He, W. Zhou, and J. Klinowski, Chem. Phys. Lett. 244, 117 (1995).
G.A. Ozin, C.T. Kresge, and H. Yang, in Mesoporous Molecular Sieves 1998, edited by L. Bonneviot, F. Béland, C. Danumah, S. Giasson, and S. Kaliaguine, Studies in Surface Science and Catalysis (Elsevier, Amsterdam, 1998), Vol. 117.
N. Coombs, D. Khushalani, S. Oliver, G.A. Ozin, G.C. Shen, I. Sokolov, and H. Yang, J. Chem. Soc., Dalton Trans. 3941 (1997).
S. Sadasivan, D. Khushalani, and S. Mann, J. Mater. Chem. 13, 1023 (2003).
G.A. Ozin, H. Yang, I. Sokolov, and N. Coombs, Adv. Mater. 9, 662 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendonza, A.M., Warzywoda, J. & Sacco, A. Investigation of structural order and morphology of MCM-41 mesoporous silica using an experimental design methodology. J Porous Mater 13, 37–47 (2006). https://doi.org/10.1007/s10934-006-5488-0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10934-006-5488-0