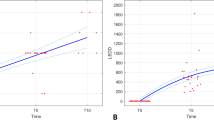
We have modelled the Unified Parkinson’s Disease Rating Scale (UPDRS) scores collected in 800 subjects followed for 8 years. Newly diagnosed and previously untreated subjects were initially randomized to treatment with placebo, deprenyl, tocopherol or both and, when clinical disability required, received one or more dopaminergic agents (levodopa (carbidopa / levodopa), bromocriptine, or pergolide). Using models for disease progression and pharmacodynamic models for drug effects we have characterized the changes in UPDRS over time to determine the influence of the various drug treatments. We have confirmed and quantitated the relative symptomatic benefits of the dopaminergic agents and provide model-based evidence for slowing of disease progression.
Similar content being viewed by others
References
Fahn S. (1999). Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier vs Later L-DOPA. Arch. Neurol. 56(5):529–535
Schulzer M., Mak E., and Calne D.B. (1992). The antiparkinson efficacy of deprenyl derives from transient improvement that is likely to be symptomatic. Ann. Neurol. 32(6):795–798
Whone A.L., Watts R.L., Stoessl A.J., Davis M., Reske S., Nahmias C. et al. (2003). Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann. Neurol. 54(1):93–101
Parkinson Study Group. (2002). Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. J. Am. Med. Assoc. 287(13):1653–1661
Davis K.L., Thal L.J., Gamzu E.R., Davis C.S., Woolson R.F., Gracon S.I. et al. (1992). A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease The Tacrine Collaborative Study Group. N. Engl. J. Med. 327(18):1253–1259
The Parkinson Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N. Engl. J. Med. 328:176–183 (1993).
Holford N.H.G., Ludden T. (1994). Time course of drug effect. In: Welling P.G., Balant L.P. (eds) Handbook of Experimental Pharmacology Chapter 11. Springer-Verlag, Heidelberg
Chan P.L.S. and Holford N.H.G. (2001). Drug treatment effects on disease progression. Annu. Rev. Pharmacol. Toxicol. 41:625–659
Holford N.H.G., Kimko H.C., Monteleone J.P., and Peck C.C. (2000). Simulation of clinical trials. Annu. Rev. Pharmacol. Toxicol. 40:209–234
The Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson’s disease. N. Engl. J. Med. 321:1364–1371 (1989).
Holford N.H.G., Mould D.R., and Peck C.C. (2001). Disease Progress Models. In: Atkinson A. (eds). Principles of Clinical Pharmacology. Academic Press, San Diego, pp. 253–262
Holford N.H.G. and Sheiner L.B. (1981). Understanding the dose-effect relationship: Clinical application of pharmacokinetic-pharmacodynamic models. Clin. Pharmacokinet. 6:429–453
Holford N.H.G. and Peace K.E. (1992). Methodologic aspects of a population pharmacodynamic model for cognitive effects in Alzheimer patients treated with tacrine. Proc. Natl. Acad. Sci. USA 89:11466–11470
Pillai G., Gieschke R., Goggin T., Jacqmin P., R.C. Schimmer, and Steimer J.L. (2004). A semimechanistic and mechanistic population PK-PD model for biomarker response to ibandronate, a new bisphosphonate for the treatment of osteoporosis. Br. J. Clin. Pharmacol. 58(6):618–631
Chan P.L.S., Nutt J.G., and Holford N.H.G. (2005). Pharmacokinetic and pharmacodynamic changes during the first four years of levodopa treatment in Parkinson’s disease. J. Pharmacokinet. Pharmacodyn. 32:459–484
The Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP patients requiring levodopa. Ann. Neurol. 39:37–45 (1996).
The Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP subjects not requiring levodopa. Ann. Neurol. 39:29–36 (1996).
The Parkinson Study Group. Mortality in DATATOP: A multicenter trial in early Parkinson’s disease. Ann. Neurol. 43(3):318–325 (1998).
Shoulson I., Oakes D., Fahn S., Lang A., Langston J.W., LeWitt P. et al. (2002). Impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson’s disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy of parkinsonism trial. Ann. Neurol. 51(5):604–612
Beal S.L., Boeckmann A.J., and L. B. Sheiner. NONMEM Project Group. In. NONMEM Users Guides V. Version (ed.). University of California at San Francisco, San Francisco, 1999.
Sheiner L.B. (1997). Learning versus confirming in clinical drug development. Clin. Pharmacol. Ther. 61(3):275–291
Pålhagen S., Heinonen E.H., Hägglund J., Kaugesaar T., Kontants H., Mäki-Ikola O. et al. (1998). Selegiline delays the onset of disability in de novo parkinsonian patients. Neurology 51:520–525
Holford N.H.G. (1997). Population models for Alzheimer’s and Parkinson’s disease. In: Aarons L. and Balant L.P. (eds) The Population Approach: Measuring and Managing Variability in Response, Concentration and Dose. COST B1 European Commission, Brussels, pp. 97–104
Contin M., Riva R., Martinelli P., Cortelli P., Albani F., and Baruzzi A. (1994). Longitudinal monitoring of the levodopa concentration-effect relationship in Parkinson’s disease. Neurology 44:1287–1292
Nutt J.G. and Holford N.H.G. (1996). The response to levodopa in Parkinson’s disease: Imposing pharmacological law and order. Ann. Neurol. 39:561–573
Nutt J.G., Carter J.H., Lea E.S., and Sexton G.J. (2002). Evolution of the response to levodopa during the first 4 years of therapy. Ann. Neurol. 51(6):686–693
Parkinson Study Group. A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch. Neurol. 61(4):561–566 (2004).
Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 351(24):2498–2508 (2004).
Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease. J. Am. Med. Assoc. 284(15):1931–1938 (2000).
Rascol O., Brooks D.J., Korczyn A.D., De Deyn P.P., Clarke C.E., and Lang A.E. (2000). A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N. Engl. J. Med. 342(20):1484–1491
Mallinckrodt C.H., Clark S.W., Carroll R.J., and Molenbergh G. (2003). Assessing response profiles from incomplete longitudinal clinical trial data under regulatory considerations. J. Biopharm. Stat. 13(2):179–190
Hu C. and Sale M.E. (2003). A joint model for nonlinear longitudinal data with informative dropout. J. Pharmacokinet. Pharmacodyn. 30(1):83–103
Muenter M.D., and Tyce G.M. (1971). L-dopa therapy of Parkinson’s disease, plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin. Proc. 46:231–239
Clarke C.E., and Davies P. (2000). Systematic review of acute levodopa and apomorphine challenge tests in the diagnosis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 69(5):590–594
Markham C.H., and Diamond S.G.(1981). Evidence to support early levodopa therapy in Parkinson disease. Neurology 31:125–131
Fahn S. (1996). Is levodopa toxic?. Neurology 47(Suppl 3):S184–S195
Murer M.G., Raisman-Vozari R. and Gershanik O. (1999). Levodopa in Parkinson’s disease: neurotoxicity issue laid to rest? Drug Saf. 21(5):339–352
Agid Y., Chase T., and Marsden D. (1998). Adverse reactions to levodopa: drug toxicity or progression of disease?. Lancet 351(9106):851–852
Murer M.G., Dziewczapolski G., Menalled L.B., Garcia M.C., Agid Y., Gershanik O. et al. (1998). Chronic levodopa is not toxic for remaining dopamine neurons, but instead promotes their recovery, in rats with moderate nigrostriatal lesions. Ann. Neurol. 43(5):561–575
Olanow C.W., Hauser R.A., Gauger L., Malapira T., Koller W., Hubble J. et al. (1995). The effect of deprenyl and levodopa on the progression of Parkinson’s disease. Ann. Neurol. 38:771–777
Hauser R.A., and Holford N.H.G. (2002). Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson’s disease. Mov. Disord. 17(5):961–968
Schapira A.H. (2003). Neuroprotection in PD-A role for dopamine agonists?. Neurology 61(6 Suppl 3):S34–42
P. Chan L.S., Nutt J.G., N. Holford H.G., and Parkinson Study Group. Application of clinical trial simulation to evaluate the ELLDOPA trial design. Mov. Disord. 17(5):S111–S112 (2002).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Holford, N.H.G., Chan, P.L.S., Nutt, J.G. et al. Disease Progression and Pharmacodynamics in Parkinson Disease – Evidence for Functional Protection with Levodopa and Other Treatments. J Pharmacokinet Pharmacodyn 33, 281–311 (2006). https://doi.org/10.1007/s10928-006-9012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-006-9012-6