Abstract
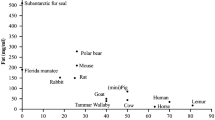
Ionized calcium ([Ca2+]) is present in milk at concentrations around 3 mM, a concentration that drives the formation of complexes with citrate, phosphate, and casein, thereby generating compounds that carry the major portion of calcium in milk. In humans and cows, where it has been studied, changes in milk calcium appear to be regulated by the amount of citrate and casein in milk rather than changes in [Ca2+]. Most or all of the calcium in milk is likely derived through exocytosis of secretory vesicles derived from the Golgi compartment where a calcium ATPase mediates transport from the cytoplasm. The identity of the transporters is not yet certain but gene expression for the plasma membrane calcium ATPase, PMCA2bw, and the secretory pathway calcium ATPase, SPCA, is highly upregulated during lactation. Currently nothing appears to be known about the mechanisms that mediate transport of calcium across the basolateral membrane of the alveolar cell.
Similar content being viewed by others
Abbreviations
- [Ca2+]:
-
concentration of ionized calcium
- ER:
-
endoplasmic reticulum
- PMCA:
-
plasma membrane calcium transporter
- PMR1:
-
yeast calcium ATPase
- SERCA:
-
sarcoplasmic reticulum/ER calcium ATPase
- SPCA:
-
secretory pathway calcium ATPase
References
Holt C, Jenness R. Interrelationships of constituents and partition of salts in milk samples from eight species. Comp Biochem Physiol 1984;77A:275–82.
Neville MC, Keller RP, Casey CE, Allen JC. Calcium partitioning in human and bovine milk. J Dairy Sci 1994;77:1964–75.
Van Houten JN. Calcium sensing by the mammary gland. J Mammary Gland Biol and Neoplasia 2005;10:In Press.
Griffin MCA, Lyster RL, Price JC. The disaggregationof calcium-depleted casein micelles. Eur J Biochem 1988;174:339–43.
Blake HH, Henning SJ. Absorption and transport of milk calcium by infant rats. Am J Physiol 1988;254:G12–G19.
Ruegg M, Blanc B. Structure and properties of the particulate constitutents of human milk: A review. Food Microstruct 1982;1:25–47.
Neville MC, Allen JC, Zhang P. Ionic Interactions in Milk. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. p. 577–92.
Lonnerdal B, Lien EL. Nutritional and physiologic significance of alpha-lactalbumin in infants. Nutr Rev 2003;61:295–305.
Segawa T, Sugai S. Interactions of divalent metal ions with bovine, human, and goat alpha-lactalbumins. J Biochem (Tokyo) 1983;93:1321–28.
Allen JC, Neville MC. Ionized calcium in human milk determined with a calcium-selective electrode. Clin Chem 1983;29:858–61.
Allen JC, Keller RP, Archer PC, Neville MC. Studies in human lactation: Milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr 1991;54:69–80.
Casey CE, Hambidge KM. Nutritional aspects of human lactation. In: Neville MC, Neifert MA, editors. Lactation: physiology, nutrition and breast-feeding. N.Y.: Plenum Press; 1983. p. 199–248.
White JCD, Davies DT. The relation between the chemical composition of milk and the stability of the caseinate complex—I. General introduction, description of samples, methods, and chemical composition of samples. J Dairy Res 1958;30:171–89.
Holt C, Dalgleish DG, Jenness R. Calculation of the ion equilibria in milk diffusate and comparison with experiment. Anal Biochem 1981;113:154–63.
Swaisgood HE. Protein and amino acid composition of bovine milk. In: Jensen RG, editor. Handbook of Milk Composition. San Diego: Academic Press; 1995. p. 464–67.
Slattery CW. Review: Casein micelle structure: An examination of models. J Dairy Sci 1976;59:1547–56.
Little EM, Holt C. An equilibrium thermodynamic model of the sequestration of calcium phosphate by casein phosphopeptides. Eur Biophys J 2004;33:435–47.
Holt C, Timmins PA, Errington N, Leaver J. A core-shell model of calcium phosphate nanoclusters stabilized by beta-casein phosphopeptides, derived from sedimentation equilibrium and small-angle X-ray and neutron-scattering measurements. Eur J Biochem 1998;252:73–78.
Kumosinski TF, Brown EM, Farrell HM, Jr. Three-dimensional molecular modeling of bovine caseins: A refined, energy-minimized kappa-casein structure. J Dairy Sci 1993;76:2507–20.
Kakalis LT, Kumosinski TF, Farrell HM, Jr. A multinuclear, high-resolution NMR study of bovine casein micelles and submicelles. Biophys Chem 1990;38:87–98.
Groves ML, Gordon WG. The major component of human casein: A protein phosphorylated at different levels. Arch Biochem Biophys 1970;140:47–51.
Jenness R. Comparative aspects of milk proteins. J Dairy Res 1979;46:197–210.
Laskey MA, Prentice A, Shaw J, Zachou T, Ceesay SM, Vasquez-Velasquez L, et al. Breast-milk calcium concentrations during prolonged lactation in British and rural Gambian mothers. Acta Paediatr Scand 1990;79:507–12.
Kirksey A, Ernst JA, Poepke JL, Tsai T-L. Influence of mineral intake and use of oral contraceptives before pregnancy on the mineral content of human colostrum and of more mature milk. Am J Clin Nutr 1979;32:30–39.
Prentice A, Barclay DV. Breast-milk calcium and phosphorus concentrations of mothers in rural Zaire. Eur J Clin Nutr 1991;45:611–17.
Butte NF, Garza C, Smith EO, Nichols BL. Macro- and trace-mineral intakes of exclusively breast-fed infants. Am J Clin Nutr 1987;45:42–48.
Dewey KG, Finley DA, Lonnerdal B. Breast milk volume and composition during late lactation (7–20 months). J Pediatr Gastroenterol Nutr 1984;3:713–20.
Karra MV, Udipi SA, Kirksey A, Roepke JLB. Changes in specific nutrients in beast milk during extended lactation. Am J Clin Nutr 1986;43:495–503.
Tanzer F, Sunel S. Calcium, magnesium and phosphorus concentrations in human milk and in sera of nursing mothers and their infants during 26 weeks of lactation. Indian Pediatr 1991;28:391–99.
Atkinson S, Alston-Mills B, Lonnerdal B, Neville MC. Major minerals and ionic constituents of human and bovine milks. In: Jensen RG, editor. Handbook of milk composition. 1st ed. San Diego: Academic Press; 1995. p. 593–622.
Patton S, Huston GE, Montgomery PA, Josephson RV. Approaches to the study of colostrum—the onset of lactation. In: Hamosh M, Goldman AS, editors. Human lactation 2:Maternal and environmental factors. N.Y.: Plenum press; 1986. p. 231–40.
Chen DC, Nommsen-Rivers L, Dewey KG, Lonnerdal B. Stress during labor and delivery and early lactation performance. Am J Clin Nutr 1998;68:335–44.
Neville MC, Allen JC, Archer PC, Casey CE, Seacat J, Keller RP, et al. Studies in human lactation: Milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr 1991;54:81–92.
Neville MC, Peaker M. The secretion of calcium and phosphorus into milk. J Physiol 1979;313:561–70.
Linzell JL, Mepham TB, Peaker M. The secretion of citrate into milk. J Physiol 1976;260:739–50.
Waugh DF, Talbot B. Equilibrium casein micelle systems. Biochemistry 1971;10(23):4153.
Clermont Y, Xia L, Rambourg A, Turner JD, Hermo L. Transport of casein submicelles and formation of the secretion granules in the Golgi apparatus of epithelial cells of the lactating mammary gland of the rat. Anat Rec 1993;235:363–73.
Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca(2+)-ATPase protein expression in mammary tissue. Am J Physiol Cell Physiol 2000;279:C1595–602.
Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem 2004;279(41):42369–73.
Ramberg C, Jr. Modeling calcium metabolism in pregnant and lactating cows. In: Subramamiam KN, Wastney ME, editors. Kinetic models of trace element and mineral metabolism during development. Boca Raton: CRC Press; 1995.
Hofer AM, Landolfi B, Debellis L, Pozzan T, Curci S. Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. EMBO J 1998;17:1986–95.
Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997;388:882–87.
Nguyen D-AD, Beeman NG, Lewis MT, Schaack J, Neville MC. Intraductal injection into the mouse mammary gland. In: Ip MM, Asch BB, editors. Methods in mammary gland biology and breast cancer research. N.Y.: Kluwer Academic/Plenum; 2000. p. 259–70.
Baumrucker CR, Keenan TW. Membranes of the mammary gland. X. ATP-dependent calcium accumulation by a Golgi apparatus-rich fraction from bovine mammary gland. Exp Cell Res 1975;90:253–60.
Neville MC, Selker F, Semple K, Watters C. ATP-dependent calcium transport by a Golgi-enriched membrane fraction from mouse mammary gland. J Memb Biol 1981;61:97–105.
Watters C. A Ca2+-stimulated adenosine triphosphatase in Golgi-enriched membranes of lactating murine mammary tissue. Biochem J 1984;224:39–45.
West DW. Energy-dependent calcium sequestration activity in a Golgi apparaus fraction derived from lactating rat mammary gland. Biochim Biophys Acta 1981;673:374–86.
Taylor RS, Jones SM, Dahl RH, Nordeen MH, Howell KE. Characterization of the Golgi complex cleared of proteins in transit and examination of calicum uptake activities. Mol Biol Cell 1997;8:1911–31.
Bingham EW, McGranaghan MB, Wickham ED, Leung CT, Farrell HM, Jr. Properties of [Ca2+ + Mg2+]-adenosine triphosphatases in the Golgi apparatus and microsomes of the lactating mammary glands of cows. J Dairy Sci 1993;76:393–400.
Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol 1999;276:C796–802.
Wuytack F, Raeymaekers L, Missiaen L. PMR1/SPCA Ca2+ pumps and the role of the Golgi apparatus as a Ca2+ store. Pflugers Arch 2003;446:148–53.
Powell JT, Brew K. Metal ion activation of galactosyltransferase. J Biol Chem 1976;251:3645–52.
Kuhn NJ, Ward S, Leong WS. Submicromolar manganese dependence of Golgi vesicular galactosyltransferase (lactose synthetase). Eur J Biochem 1991;195:243–50.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neville, M.C. Calcium Secretion into Milk. J Mammary Gland Biol Neoplasia 10, 119–128 (2005). https://doi.org/10.1007/s10911-005-5395-z
Issue Date:
DOI: https://doi.org/10.1007/s10911-005-5395-z