Abstract
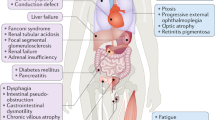
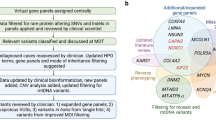
Mitochondrial next generation sequencing (NGS) panels offer single-step analysis of the numerous nuclear genes involved in the structure, function, and maintenance of mitochondria. However, the complexities of mitochondrial biology and genetics raise points for consideration in clinical use of these tests. To understand the current status of mitochondrial genetic testing, we assessed the gene offerings and consent forms of mitochondrial NGS panels available from seven US-based clinical laboratories. The NGS panels varied markedly in number of genes (101–1204 genes), and the proportion of genes causing “classic” mitochondrial diseases and their phenocopies ranged widely between labs (18 %–94 % of panel contents). All panels included genes not associated with classic mitochondrial diseases (6 %–28 % of panel contents), including genes causing adult-onset neurodegenerative disorders, cancer predisposition, and other genetic syndromes or inborn errors of metabolism. Five of the panels included genes that are not listed in OMIM to be associated with a disease phenotype (5 %–49 % of panel contents). None of the consent documents reviewed had options for patient preference regarding receipt of incidental findings. These findings raise points of discussion applicable to mitochondrial diagnostics, but also to the larger arenas of exome and genome sequencing, including the need to consider the boundaries between clinical and research testing, the necessity of appropriate informed consent, and the responsibilities of clinical laboratories and clinicians. Based on these findings, we recommend careful evaluation by laboratories of the genes offered on NGS panels, clear communication of the predicted phenotypes, and revised consent forms to allow patients to make choices about receiving incidental findings. We hope that our analysis and recommendations will help to maximize the considerable clinical utility of NGS panels for the diagnosis of mitochondrial disease.


Similar content being viewed by others
References
ACMG Board of Directors. (2012). Points to consider in the clinical application of genomic sequencing. Genetics in Medicine, 14(8), 759–761. doi:https://doi.org/10.1038/gim.2012.74.
Al-Chalabi, A., Jones, A., Troakes, C., King, A., Al-Sarraj, S., & van den Berg, L. H. (2012). The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathologica, 124(3), 339–352. doi:https://doi.org/10.1007/s00401-012-1022-4.
Barletta, J. A., & Hornick, J. L. (2012). Succinate dehydrogenase-deficient tumors: diagnostic advances and clinical implications. Advances in Anatomic Pathology, 19(4), 193–203. doi:https://doi.org/10.1097/PAP.0b013e31825c6bc6.
Bernier, F. P., Boneh, A., Dennett, X., Chow, C. W., Cleary, M. A., & Thorburn, D. R. (2002). Diagnostic criteria for respiratory chain disorders in adults and children. Neurology, 59(9), 1406–1411.
Biesecker, L. G. (2012). Opportunities and challenges for the integration of massively parallel genomic sequencing into clinical practice: lessons from the ClinSeq project. Genetics in Medicine, 14(4), 393–398. doi:https://doi.org/10.1038/gim.2011.78.
Calvo, S. E., & Mootha, V. K. (2010). The mitochondrial proteome and human disease. Annual Review of Genomics and Human Genetics, 11, 25–44. doi:https://doi.org/10.1146/annurev-genom-082509-141720.
Calvo, S. E., Compton, A. G., Hershman, S. G., Lim, S. C., Lieber, D. S., Tucker, E. J., et al. (2012). Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Science Translational Medicine, 4(118), 118ra110. doi:https://doi.org/10.1126/scitranslmed.3003310.
Delonlay, P., Rötig, A., & Sarnat, H. B. (2013). Respiratory chain deficiencies. Handbook of Clinical Neurology, 113, 1651–1666. doi:https://doi.org/10.1016/B978-0-444-59565-2.00033-2.
DiMauro, S., & Schon, E. A. (2003). Mitochondrial respiratory-chain diseases. New England Journal of Medicine, 348(26), 2656–2668. doi:https://doi.org/10.1056/NEJMra022567.
Goldstein, A. C., Bhatia, P., & Vento, J. M. (2013). Mitochondrial disease in childhood: nuclear encoded. Neurotherapeutics, 10(2), 212–226. doi:https://doi.org/10.1007/s13311-013-0185-6.
Green, R. C., Berg, J. S., Grody, W. W., Kalia, S. S., Korf, B. R., Martin, C. L., et al. (2013). ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine. doi:https://doi.org/10.1038/gim.2013.73.
Jamal, S. M., Yu, J. H., Chong, J. X., Dent, K. M., Conta, J. H., Tabor, H. K., et al. (2013). Practices and policies of clinical exome sequencing providers: analysis and implications. American Journal of Medical Genetics Part A. doi:https://doi.org/10.1002/j.1552-4833.2013.35942.x.
Kirby, D. M., & Thorburn, D. R. (2008). Approaches to finding the molecular basis of mitochondrial oxidative phosphorylation disorders. Twin Research and Human Genetics, 11(4), 395–411. doi:https://doi.org/10.1375/twin.11.4.395.
McCormick, E., Place, E., & Falk, M. J. (2013). Molecular genetic testing for mitochondrial disease: from one generation to the next. Neurotherapeutics, 10(2), 251–261. doi:https://doi.org/10.1007/s13311-012-0174-1.
Nunnari, J., & Suomalainen, A. (2012). Mitochondria: in sickness and in health. Cell, 148(6), 1145–1159. doi:https://doi.org/10.1016/j.cell.2012.02.035.
Pagliarini, D. J., Calvo, S. E., Chang, B., Sheth, S. A., Vafai, S. B., Ong, S. E., et al. (2008). A mitochondrial protein compendium elucidates complex I disease biology. Cell, 134(1), 112–123. doi:https://doi.org/10.1016/j.cell.2008.06.016.
Rosen, D. R. (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature, 364(6435), 362. doi:https://doi.org/10.1038/364362c0.
Ross, L. F., Saal, H. M., David, K. L., Anderson, R. R., & American Academy of Pediatrics; American College of Medical Genetics and Genomics. (2013). Technical report: ethical and policy issues in genetic testing and screening of children. Genetics in Medicine, 15(3), 234–245. doi:https://doi.org/10.1038/gim.2012.176.
Skladal, D., Halliday, J., & Thorburn, D. R. (2003). Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain, 126(Pt 8), 1905–1912. doi:https://doi.org/10.1093/brain/awg170.
Tucker, E. J., Compton, A. G., & Thorburn, D. R. (2010). Recent advances in the genetics of mitochondrial encephalopathies. Current Neurology and Neuroscience Reports, 10(4), 277–285. doi:https://doi.org/10.1007/s11910-010-0112-8.
Vento, J. M., & Pappa, B. (2013). Genetic counseling in mitochondrial disease. Neurotherapeutics, 10(2), 243–250. doi:https://doi.org/10.1007/s13311-012-0173-2.
Wong, L. J. (2013). Next generation molecular diagnosis of mitochondrial disorders. Mitochondrion, 13(4), 379–387. doi:https://doi.org/10.1016/j.mito.2013.02.001.
Acknowledgments
Many thanks to the clinical laboratories, whose hard work provides valuable diagnostic testing options for patients with rare genetic diseases. Thanks to Monisha Shah for her assistance with statistics.
Declarations
Julia Platt, Rachel Cox, and Gregory Enns declare that they have no conflict of interest. No animal or human studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 94 kb)
Rights and permissions
About this article
Cite this article
Platt, J., Cox, R. & Enns, G.M. Points to Consider in the Clinical Use of NGS Panels for Mitochondrial Disease: An Analysis of Gene Inclusion and Consent Forms. J Genet Counsel 23, 594–603 (2014). https://doi.org/10.1007/s10897-013-9683-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-013-9683-2