Abstract
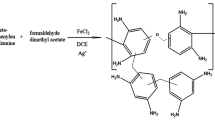
A new fluorescent probe 3, has been developed for the detection of Fe(III) in water based samples. The design of 3 involved the incorporation of Fe(III) binding sites observed in naturally occurring Siderophores into a synthetic sensing assembly. The probe, containing two Schiff base receptors connected to a mesitylene platform, was prepared in two steps. The dipodal sensor displayed good selectivity for Fe(III) when tested against other physiological and environmentally important metal ions, in HEPES buffered solution at pH 7.0, through a quenching of the fluorescent intensity. Stern-Volmer analysis of this quenching interaction indicated a 1:1 (host : guest) binding stoichiometry between the probe and Fe(III). The association constant, K a calculated using the Benesi-Hildebrand equation was found to be 3.8 × 104 M−1. Crucially, the sensor was capable of measuring Fe(III) competitively in solutions containing both Fe(III) and Cu(II). Thus, the adoption of Fe(III) binding sites found in nature, into synthetic luminescent assemblies has proven an effective design strategy for the development of new Fe(III) probes.





Similar content being viewed by others
References
Lynch SR (1997) Interaction of iron with other nutrients. Nutr Rev 55:102–110
Al-Karadaghi S, Hansson M, Nikonov S, Jönsson B, Hederstedt L (1997) Crystal structure of ferrochelatase: the terminal enzyme in heme biosynthesis. Structure 11:1501–1510 doi:10.1016/S0969-2126(97)00299-2
Mitchell MS, Walker DL, Whelan J, Bosnich B (1987) Biological analogs: synthetic iron(III)-specific chelators based on the natural siderophores. Inorg Chem 26:396–400 doi:10.1021/ic00250a012
Franchini M, Veneri D (2004) Iron-chelation therapy: an update. Hematol J 5:287–292 doi:10.1038/sj.thj.6200407
Jung HJ, Singh N, Jang DO (2008) Highly Fe(III) selective ratiometric fluorescent probe based on imine-linked benzimidazole. Tetrahedron Lett 49:2960–2964 doi:10.1016/j.tetlet.2008.03.002
Bricks JL, Kovalchuk A, Trieflinger C, Nofz M, Buschel M, Tolmachev AI, Daub J, Rurack K (2005) On the development of sensor molecules that display Fe(III)-amplified fluorescence. J Am Chem Soc 127:13522–13529 doi:10.1021/ja050652t
Ouchetto H, Dias M, Mornet R, Lesuisse E, Camadro JM (2005) A new route to trihydroxamate-containing artificial siderophores and synthesis of a new fluorescent probe. Bioorg Med Chem 13:1799–1803 doi:10.1016/j.bmc.2004.11.053
Tumambac GE, Rosencrance CM, Wolf C (2004) Selective metal ion recognition using a fluorescent 1,8-diquinolylnaphthalene-derived sensor in aqueous solution. Tetrahedron 60:11293–11297 doi:10.1016/j.tet.2004.07.053
Ma Y, Luo W, Quinn PJ, Liu Z, Hider RC (2004) Design, synthesis, physicochemical properties, and evaluation of novel iron chelators with fluorescent sensors. J Med Chem 47:6349–6362 doi:10.1021/jm049751s
Kikkeri R, Traboulsi H, Humbert N, Gumienna-Kontecka E, Arad-Yellin R, Melman G, Elhabiri M, Albrecht-Gary A-M, Shanzer A (2007) Toward iron sensors: bioinspired tripods based on fluorescent phenol-oxazoline coordination sites. Inorg Chem 46:2485–2497 doi:10.1021/ic061952u
Meijler MM, Arad-Yellin R, Cabantchik ZI, Shanzer A (2002) Synthesis and evaluation of iron chelators with masked hydrophilic moieties. J. Am. Chem. Soc. 124:12666–12667 doi:10.1021/ja027013s
Palanche T, Marmolle F, Abdallah MA, Shanzer A, Albrecht-Gary A-M (1999) Fluorescent siderophore-based chemosensors: iron(III) quantitative determinations. J. Biol. Inorg Chem 4:188–198 doi:10.1007/s007750050304
Schilt AA, Taylor PJ (1970) Simultaneous determination of iron and copper by a new spectrophotometric method. Anal Chem 32:220–224 doi:10.1021/ac60284a027
Mykytiuk AP, Russell DS, Sturgeon RE (1980) Simultaneous determination of iron, cadmium, zinc, copper, nickel, lead, and uranium in sea water by stable isotope dilution spark source mass spectrometry. Anal Chem 52:1281–1283 doi:10.1021/ac50058a029
Taylor AB, Stoj CS, Ziegler L, Kosman DJ, Hart PJ (2005) The copper-iron connection in biology: structure of the metallo-oxidase Fet3p. Proc Natl Acd Sci 102:15459–15464 doi:10.1073/pnas.0506227102
Blomberg MRA, Siegbahn PEM, Wikstrom M (2003) Metal-bridging mechanism for O-O bond cleavage in Cytochrome c oxidase. Inorg Chem 42:5231–5243 doi:10.1021/ic034060s
Singh N, Mulrooney RC, Kaur N, Callan JF (2008) A nanoparticle based chromogenic chemosensor for the simultaneous detection of multiple analytes. Chem Commun (Camb.) 4900–4902 doi:10.1039/b813423e
Perkins DF, Lindoy LF, McAuley A, Meehan GV, Turner P (2006) Manganese(II), iron(II), cobalt(II), and copper(II) complexes of an extended inherently chiral tris-bipyridyl cage. Prog Nat Acd Sci 103:532–537 doi:10.1073/pnas.0508539103
Hickford SJH, Küpper FC, Zhang G, Carrano CJ, Blunt JW, Butler A (2004) Petrobactin Sulfonate, a new siderophore produced by the marine bacterium marinobacter hydrocarbonoclasticus. J Nat Prod 67:1897–1899 doi:10.1021/np049823i
Albrecht-Gary A-M, Crumbliss AL (1998) Coordination chemistry of siderophores: thermodynamics and kinetics of iron chelation and release. Met Ions Biol Syst 35:239–327
Abergel RJ, Raymond KN (2006) Synthesis and thermodynamic evaluation of mixed hexadentate linear iron chelators containing hydroxypyridinone and terephthalamide units. Inorg Chem 45:3622–3631 doi:10.1021/ic052111a
Jurchen KMC, Raymond KN (2006) A bidentate terephthalamide ligand, TAMmeg, as an entry into terephthalamide-containing therapeutic iron chelating agents. Inorg Chem 45:2438–2447 doi:10.1021/ic051287+
Barbeau K, Zhang G, Live DH, Butler A (2002) Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J Am Chem Soc 124:378–379 doi:10.1021/ja0119088
Bergeron RJ, Huang G, Smith RE, Bharti N, McManis JS, Butler A (2003) Total synthesis and structure revision of petrobactin. Tetrahedron 59:2007–2014 doi:10.1016/S0040-4020(03)00103-0
Mitchell JM, Shaw JT (2007) Synthesis and stereochemical assignment of Brasilibactin A. Org Lett 9:1679–1681 doi:10.1021/ol070355o
Ito Y, Ishida K, Okada S, Murakami M (2004) The absolute stereochemistry of anachelins, siderophores from the cyanobacterium Anabaena cylindrica. Tetrahedron 60:9075–9080 doi:10.1016/j.tet.2004.07.105
Ino A, Murabayashi A (2001) Synthetic studies of thiazoline and thiazolidine-containing natural products. Part 3: total synthesis and absolute configuration of the siderophore Yersiniabactin. Tetrahedron 57:1897–1902
Zamri A, Abdallah MA (2000) An improved stereocontrolled synthesis of Pyochelin, siderophore of Pseudomonas aeruginosa and Burkholderia cepacia. Tetrahedron 56:249–256 doi:10.1016/S0040-4020(99)00946-1
Van der Made AW, Van der Made RH (1993) A convenient procedure for bromomethylation of aromatic compounds. Selective mono-, bis-, or trisbromomethylation. J Org Chem 58:1262–1263 doi:10.1021/jo00057a046
Singh N, Hundal MS, Hundal G, Martinez-Ripoll M (2005) Zinc templated synthesis—a route to get metal ion free tripodal ligands and lariat coronands, containing Schiff bases. Tetrahedron 61:7796–7806 doi:10.1016/j.tet.2005.05.052
Bhardwaj VK, Singh N, Hundal MS, Hundal G Mesitylene based azo-coupled chromogenic tripodal receptors—a visual detection of Ag(I) in aqueous medium. Tetrahedron 6:7878–7886 (▪▪▪) doi:10.1016/j.tet.2006.05.047
Hadjoudis E, Mavridis IM (2004) Photochromism and thermochromism of Schiff bases in the solid state: structural aspects. Chem Soc Rev 33:579–588
Singh N, Mulrooney RC, Kaur N, Callan JF (2008) A ratiometric fluorescent probe for magnesium employing excited state intramolecular proton transfer. Tetrahedron Lett 49:6690–6692 doi:10.1016/j.tetlet.2008.09.052
Galindo F, Becerril M, Burgette I, Luis SV, Viagra L (2004) Synthesis and study of a cyclophane displaying dual fluorescence emission: a novel ratiometric sensor for carboxylic acids in organic medium. Tetrahedron Lett 45:1659–1662 doi:10.1016/j.tetlet.2003.12.116
Callan JF, de Silva AP, Magri DC (2005) Luminescent sensors and switches in the early 21st century. Tetrahedron 61:8551–8588 doi:10.1016/j.tet.2005.05.043
Fabbrizzi L, Poggi A (1995) Sensors and switches from supramolecular chemistry. Chem Soc Rev 24:197 doi:10.1039/cs9952400197
Mei X, Wolf C (2004) Enantioselective sensing of chiral carboxylic acids. J Am Chem Soc 126:14736–14737 doi:10.1021/ja0459781
Job P (1928) Formation and stability of inorganic complexes in solution. Ann Chim 9:113–203
Benesi HA, Hilderbrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2704
Acknowledgements
The authors would like to acknowledge financial assistance from the EPSRC and RGU. They also acknowledge the EPSRC national mass spectrometry service in Swansea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the linked to the electronic supplementary material
Supplementary data
(PDF 369 kb)
Rights and permissions
About this article
Cite this article
Singh, N., Kaur, N. & Callan, J.F. Incorporation of Siderophore Binding Sites in a Dipodal Fluorescent Sensor for Fe(III). J Fluoresc 19, 649–654 (2009). https://doi.org/10.1007/s10895-008-0457-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-008-0457-4