Abstract
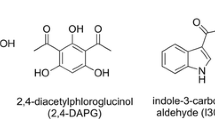
Disease has spurred declines in global amphibian populations. In particular, the fungal pathogen Batrachochytrium dendrobatidis has decimated amphibian diversity in some areas unaffected by habitat loss. However, there is little evidence to explain how some amphibian species persist despite infection or even clear the pathogen beyond detection. One hypothesis is that certain bacterial symbionts on the skin of amphibians inhibit the growth of the pathogen. An antifungal strain of Janthinobacterium lividum, isolated from the skin of the red-backed salamander Plethodon cinereus, produces antifungal metabolites at concentrations lethal to B. dendrobatidis. Antifungal metabolites were identified by using reversed phase high performance liquid chromatography, high resolution mass spectrometry, nuclear magnetic resonance, and UV-Vis spectroscopy and tested for efficacy of inhibiting the pathogen. Two metabolites, indole-3-carboxaldehyde and violacein, inhibited the pathogen’s growth at relatively low concentrations (68.9 and 1.82 μM, respectively). Analysis of fresh salamander skin confirmed the presence of J. lividum and its metabolites on the skin of host salamanders in concentrations high enough to hinder or kill the pathogen (51 and 207 μM, respectively). These results support the hypothesis that cutaneous, mutualistic bacteria play a role in amphibian resistance to fungal disease. Exploitation of this biological process may provide long-term resistance to B. dendrobatidis for vulnerable amphibians and serve as a model for managing future emerging diseases in wildlife populations.



Similar content being viewed by others
References
Belden, L. K., and Harris, R. N. 2007. Infectious diseases in wildlife: the community ecology context. Front. Ecol. Environ. 5:533–539.
Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., Slocombe, R., Ragan, M. A., Hyatt, A. D., Mcdonald, K. R., Hines, H. B., Lips, K. R., Marantelli, G., and Parkes, H. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. U. S. A. 95:9031–9036.
Briggs, C. J., Vredenburg, V. T., Knapp, R. A., and Rachowicz, L. J. 2005. Investigating the population-level effects of chytridiomycosis: an emerging infectious disease of amphibians. Ecology 86:3149–3159.
Brizzi, R., Delfino, G., and Pellegrini, R. 2002. Specialized mucous glands and their possible adaptive role in the males of some species of Rana (Amphibia, Anura). J. Morphol. 254:328–341.
Brucker, R. M., Baylor, C. M., Walters, R. L., Lauer, A., Harris, R. N., and Minbiole, K. P. C. 2008. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J. Chem. Ecol. 43:39–43.
Currie, C. R., Poulsen, M., Mendenhall, J., Boomsma, J. J., and Billen, J. 2006. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311:81–83.
Daszak, P., Strieby, A., Cunningham, A. A., Longcore, J. E., Brown, C. C., and Porter, D. 2004. Experimental evidence that the bullfrog (Rana catsbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol. J. 14:201–207.
Ducklow, H. W., and Mitchell, R. 1979. Bacterial populations and adaptations in the mucus layers on living corals. Limnol. Oceanogr. 24:715–725.
Durán, N., and Menck, C. F. M. 2001. Chromobacterium violaceum: A review of pharmacological and Industrial perspectives. Crit. Rev. Microbiol. 27:201–222.
Gutierrez-Lugo, M.-T., Woldemichael, G. M., Singh, M. P., Suarez, P. A., Maiese, W. M., Montenegro, G., and Timmermann, B. N. 2005. Isolation of three new naturally occurring compounds from the culture of Micromonospora sp. P1068. Nat. Prod. Res. 19:645–652.
Haas, D., and Degafo, G. 2005. Biological control of soil-borne pathogens by fluorescent Pseudomonas. Nat. Rev. Microbiol. 3:307–319.
Harris, R. N., James, T. Y., Lauer, A., Simon, M. A., and Patel, A. 2006. The amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3:53–56.
Lauer, A., Simon, M. A., Banning, J. L., André, E., Duncan, K., and Harris, R. N. 2007. Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia 3:630–640.
Lauer, A., Simon, M. A., Banning, J. L., Lam, B., and Harris, R. N. 2008. Diversity of cutaneous bacteria with antifungal activity isolated from the female four-toed salamanders. Int. Soc. Microb. Ecol. 2:145–157.
Lips, K. R. 1999. Mass mortality and population declines of anurans at an upland site in western Panama. Conserv. Biol. 13:117–125.
Lips, K. R., Brem, F., Brenes, R., Reeve, J. D., Alford, R. A., Voyles, J., Carey, C., Livo, L., Pressier, A. P., and Collins, J. P. 2006. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl. Acad. Sci. U. S. A. 103:3165–3170.
Matz, C., Webb, J. S., Schupp, P. J., Phang, S. Y., Penesyan, A., Egan, S., Steinberg, P., and Kjelleberg, S. 2008. Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. Pub. Libr. Sci. U. S. A. 7:3e2744.
Mcfall-Ngai, M. J. 1999. Consequences of evolving with bacterial symbionts: insights from the squid–Vibrio associations. Annu. Rev. Ecol. Syst. 30:235–256.
Rachowicz, L. J., Knapp, R. A., Morgan, J. A., Stice, M. J., Vredenburg, V. T., Parker, J. M., and Briggs, C. J. 2006. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87:1671–1683.
Rollins-Smith, L. A., Reinert, L. K., Miera, V., and Conlon, J. M. 2002. Antimicrobial peptide defenses of the Tarahumara frog, Rana tarahumarae. Biochem. Biophys. Res. Comm. 297:361–367.
Simmaco, M., Mignogna, G., and Barra, D. 1998. Antimicrobial peptides from amphibian skin: What do they tell us? Biopolymers 47:435–450.
Stuart, S. N., Chanson, J. S., Cox, N. A., Young, B. E., Rodrigues, A. S. L., Fischman, D. L., and Waller, R. W. 2004. Status and trends of amphibian declines and extinctions wordwide. Science (Wash.) 306:1783–1786.
Woodhams, D. C., Ardipradja, K., Alford, R. A., Marantelli, G., Reinert, L. K., and Rollins-Smith, L. A. 2007a. Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim. Conserv. 10:409–417.
Woodhams, D. C., Vredenburg, V. T., Stice, M. J., Simon, M. A., Billheimer, D., Shakhtour, B., Shyr, Y., Briggs, C. J., Rollins-Smith, L. A., and Harris, R. N. 2007b. Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biol. Conserv. 138:390–398.
Young, S., Berger, L., and Spear, R. 2007. Amphibian chytridiomycosis: strategies for captive management and conservation. Int. Zoo. Yb. 41:85–95.
Yue, Q., Miller, C. J., White, J. F., and Richardson, M. D. 2000. Isolation and characterization of fungal inhibitors from Epichloe festucae. J. Agric. Food Chem. 48:4687–4692.
Acknowledgements
This work was supported by a Research Corporation Cottrell College Science Award (KPCM), the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust (KPCM), a National Science Foundation Research in Undergraduate Institutions grant 0640373 (RNH), and James Madison University. HR-MS analyses were conducted by the Mass Spectrometry Facility at Harvard University (Cambridge, MA, USA). The authors would like to thank Dr. Nancy Staub for mucus depth discussions and Dr. Grace Wyngaard for a critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
RP-HPLC UV-Vis spectra of Janthinobacterium lividum extract, indole-3-carboxaldehyde standard, and violacein standard. UV-Vis spectra of (a) indole-3-carboxaldehyde and (b) violacein. Dashed lines refer to standards and solid lines refer to extracts from J. lividum. For both standards and extracts, the RP-HPLC retention time of indole-3-carboxaldehyde was approximately 9.06 min and violacein was approximately 11.15 min. (DOC 143 KB)
Supplementary Figure 2
A composite* of 16S rRNA gene DGGE profiles of skin bacteria from the wild caught salamanders, Plethodon cinereus, and comparison with migration of 16S rRNA gene fragments of Janthinobacterium lividum. The J. lividum standard (right lane) run along with the wild-caught salamanders and the resulting bands were matched (indicated with an arrow). *A composite of two separate gels with the same standard for comparison. Lane one is superimposed from an analogous DGGE. (DOC 19.5 KB)
Supplementary Table 1
1H NMR (600 MHz) and HR-MS analysis of antifungal compounds isolated from a broth culture of Janthinobacterium lividum. (DOC 26.5 KB)
Supplementary Table 2
Skin mucus depth determinations of Plethodon cinereus. a (DOC 61.5 KB)
Rights and permissions
About this article
Cite this article
Brucker, R.M., Harris, R.N., Schwantes, C.R. et al. Amphibian Chemical Defense: Antifungal Metabolites of the Microsymbiont Janthinobacterium lividum on the Salamander Plethodon cinereus . J Chem Ecol 34, 1422–1429 (2008). https://doi.org/10.1007/s10886-008-9555-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9555-7