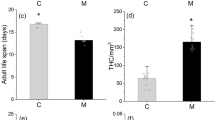
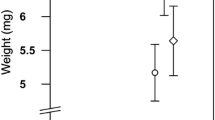
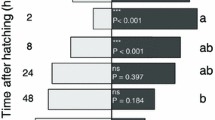
Abstract
Plants are able to “notice” insect egg deposition and to respond by activating direct and indirect defenses. An overview of these defenses and the underlying mechanisms is given from a tritrophic perspective. First, the interface between plant and eggs is addressed with respect to the mode of attachment of eggs on the plant surface. It is elucidated which plant cells might respond to components from insect eggs or the egg deposition. The scarce knowledge on the elicitors associated with the eggs or the egg-laying female is outlined. Since endosymbiotic microorganisms are often present on the eggs, and microorganisms are also abundant on the leaf surface, the role of these hidden players for eliciting oviposition-induced plant responses is considered. Furthermore, the question of which physiological and molecular processes are induced within the plant in response to egg deposition is addressed. Second, studies on the response of the herbivorous insect to oviposition-induced plant defenses are outlined. Third, the importance of oviposition-induced plant volatiles and contact cues for host and prey location of parasitoids and predators is discussed in the context of other informative chemicals used by carnivores when searching for food. Finally, physiological and ecological costs of oviposition-induced plant responses are addressed.

Similar content being viewed by others
References
Agrawal, A. A., Tuzun, S., and Bent, E. 1999. Induced Plant Defenses Against Pathogens and Herbivores. APS Press, St. Paul, MN.
Alborn, H. T., Turlings, T. C. J., Jones, T. H., Stenhagen, G., Loughrin, J. H., and Tumlinson, J. H. 1997. An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949.
Andersson, J., Borg-Karlson, A.-K., and Wiklund, C. 2003. Antiaphrodisiacs in pierid butterflies: a theme of variation. J. Chem. Ecol. 29:1489–1499.
Arimura, G.-I., Ozawa, R., Shimoda, T., Nishioka, T., Boland, W., and Takabayashi, J. 2000. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515.
Balbyshev, N. F. and Lorenzen, J. H. 1997. Hypersensitivity and egg drop, a novel mechanism of host-plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 90:652–657.
Bjorksten, T. A. and Hoffmann, A. A. 1998. Persistence of experience effects in the parasitoid Trichogramma nr brassicae. Ecol. Entomol. 23:110–117.
Blaakmeer, A., Hagenbeek, D., Van Beek, T. A., de Groot, A. E., Schoonhoven, L. M., and Van Loon, J. J. A. 1994. Plant response to eggs vs. host marking pheromone as factors inhibiting oviposition by Pieris brassicae. J. Chem. Ecol. 20:1657–1665.
Bolter, C. J., Dicke, M., Van Loon, J. J. A., Visser, J. H., and Posthumus, M. A. 1997. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol. 23:1003–1023.
Bown, A. W., Hall, D. E., and MacGregor, K. B. 2002. Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol. 129:1430–1434.
Clausen, C. P. 1976. Phoresy among entomophagous insects. Annu. Rev. Entomol. 21:343–368.
Colazza, S., Salerno, G., and Waijnberg, E. 1999. Volatiles and contact chemicals released by Nezara viridula (Heteroptera: Pentatomidae) have a kairomonal effect on the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). Biol. Control 16:310–317.
Colazza, S., Fucarino, A., Peri, E., Salerno, G., Conti, E., and Bin, F. 2004a. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 207:47–53.
Colazza, S., McElfresh, J. S., and Millar, J. G. 2004b. Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J. Chem. Ecol. 30:945–964.
De Jong, E. J. and Pak, G. A. 1984. Factors determining differential host egg recognition of two host species by different Trichogramma sp. Meded. Fac. Landbouwwet. Rijksuniv. Gent. 49:815–825.
De Moraes, C. M., Mescher, M. C., and Tumlinson, J. H. 2001. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:577–580.
Dicke, M. and Bruin, J. 2001. Chemical information transfer between wounded and unwounded plants: back to the future. Biochem. Syst. Ecol. 29:981–995.
Dicke, M. and Hilker, M. 2003. Induced plant defences: from molecular biology to evolutionary ecology. Basic Appl. Ecol. 4:3–14.
Dicke, M. and Van Loon, J. A. A. 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97:237–249.
Dickinson, C. H. and Preece, T. F. 1976. Microbiology of Aerial Plant Surfaces. Academic Press, London.
Dirie, A. M. and Gabriel, B. P. 1998. The effect of semiochemicals on efficiency and parasitism of Trichogramma evanescens (Westwood), an egg parasitoid of Asian corn borer. Asia Life Sci. 7:131–140.
Doss, R. P., Proebsting, W. M., Potter, S. W., Clement, S. L., and Williamson, R. T. 1995. Response of Np mutant of pea (Pisum sativum L.) to pea weevil (Bruchus pisorum L.) oviposition and extracts. J. Chem. Ecol. 21:97–106.
Doss, R. P., Oliver, J. E., Proebsting, W. M., Potter, S. W., Kuy, S. R., Clement, S. L., Williamson, R. T., Carney, J. R., and Devilbiss, E. D. 2000. Bruchins-insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA 97:6218–6223.
Engelberth, J., Koch, T., Kühnemann, F., and Boland, W. 2000. Ionenkanalbildende Peptaibole sind hochwirksame Elicitoren des pflanzlichen Sekundärstoffwechsels und der Rankenkrümmung. Angew. Chem. 112:1928–1930.
Fatouros, N. E., Bukovinszkine Kiss, G., Kalkers, L. A., Soler Gamborena, R., Dicke, M., and Hilker, M. 2005a. Plant synomone induced by butterfly eggs arrests Trichogramma wasps. Entomol. Exp. Appl. 115:207–215.
Fatouros, N. E., Huigens, M. E., Van Loon, J. J. A., Dicke, M., and Hilker, M. 2005b. Riders on the storm: hitch-hiking parasitic wasps spy on butterfly anti-aphrodisiac. Nature 433:704.
Felton, G. W. and Eichenseer, H. 1999. Herbivore saliva and its effects on plant defense against herbivores and pathogens, pp. 19–36, in A. A. Agrawal, S. Tuzun, and E. Bent (eds.). Induced Plant Defenses Against Pathogens and Herbivores. APS Press, St. Paul, MN.
Fukushima, J., Kainoh, Y., Honda, H., and Takabayashi, J. 2002. Learning of herbivore-induced and nonspecific plant volatiles by a parasitoid, Cotesia kariyai. J. Chem. Ecol. 28:579–586.
Hall, D. E., MacGregor, K. B., Nijsse, J., and Bown, A. W. 2004. Footsteps from insect larvae damage leaf surfaces and initiate rapid responses. Eur. J. Plant Pathol. 110:441–447.
Harari, A. R., Ben-Yakir, D., and Rosen, D. 1994. Mechanism of aggregation behavior in Maladera matrida Argaman (Coleoptera: Scarabaeidae). J. Chem. Ecol. 20:361–371.
Herms, D. A. and Mattson, W. J. 1992. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67:283–335.
Hilker, M. 1994. Egg deposition and protection of eggs in Chrysomelidae, pp. 263–276, in P. Jolivet, M. L. Cox, and E. Petitpierre (eds.). Novel Aspects of Biology of Chrysomelidae. Kluwer Academic Publishers, Dordrecht.
Hilker, M. and Meiners, T. 2002. Induction of plant responses towards oviposition and feeding of herbivorous arthropods: a comparison. Entomol. Exp. Appl. 104:181–192.
Hilker, M., Blaeske, V., Kobs, C., and Dippel, C. 2000. Kairomonal effects of sawfly sex pheromones on egg parasitoids. J. Chem. Ecol. 26:221–231.
Hilker, M., Kobs, C., Varama, M., and Schrank, K. 2002a. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J. Exp. Biol. 205:455–461.
Hilker, M., Rohfritsch, O., and Meiners, T. 2002b. The plant's response towards insect oviposition, pp. 205–234, in M. Hilker and T. Meiners (eds.). Chemoecology of Insect Eggs and Egg Deposition. Blackwell, Berlin.
Hilker, M., Stein, C., Schröder, R., Varama, M., and Mumm, R. 2005. Insect egg deposition induced defence response in Pinus sylvestris. Characterization of the elicitor. J. Exp. Biol. 208:1849–1854.
Horiuchi, J.-I., Arimura, G.-I., Ozawa, R., Shimoda, T., Takabayashi, J., and Nishioka, T. 2003. A comparison of the responses of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to volatiles emitted from lima bean leaves with different levels of damage made by T. urticae or Spodoptera exigua (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 38:109–116.
Ignacimuthu, S., Wäckers, F. L., and Dorn, S. 2000. The role of chemical cues in host finding and acceptance by Callosobruchus chinensis. Entomol. Exp. Appl. 96:213–219.
Kalberer, N., Turlings, T. C. J., and Rahier, M. 2001. Attraction of a leaf beetle (Oreina cacaliae) to damaged host plants. J. Chem. Ecol. 27:647–661.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. Chicago University Press, Chicago.
Kelling, F. J., Ialenti, F., and Den Otter, C. J. 2002. Background odour induces adaptation and sensitization of olfactory receptors in the antennae of houseflies. Med. Vet. Entomol. 16:161–169.
Kellner, R. L. L. 2002. The role of microorganisms for eggs and progeny, pp. 149–170, in M. Hilker and T. Meiners (eds.). Chemoecology of Insect Eggs and Egg Deposition. Blackwell, Berlin.
Kessler, A. and Baldwin, I. T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144.
Kessler, A. and Baldwin, I. T. 2002. Plant responses to insect herbivory: the emerging molecular analysis. Annu. Rev. Plant Biol. 53:299–328.
Kinkel, L. L. 1997. Microbial population dynamics on leaves. Annu. Rev. Phytopathol. 35:327–347.
Landolt, P. J. 1993. Effects of host plant leaf damage on cabbage looper moth attraction and oviposition. Entomol. Exp. Appl. 67:79–85.
Lait, C. G., Albron, H. T., Teal, P. E. A., and Tumlinson, J. H. 2003. Rapid biosynthesis of N-linolenoyl-l-glutamine, an elicitor of plant volatiles, by membrane-associated enzymes in Manduca sexta. Proc. Natl. Acad. Sci. USA 100:7027–7032.
Loughrin, J. H., Potter, D. A., Hamilton, K. T., and Byers, M. E. 1996. Role of feeding-induced plant volatiles in aggregative behavior of the Japanese beetle (Coleoptera: Scarabaeidae). Environ. Entomol. 25:1188–1191.
Maffei, M., Bossi, S., Spiteller, D., Mithöfer, A., and Boland, W. 2004. Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol. 134:1752–1762.
McGregor, R. and Henderson, D. 1998. The influence of oviposition experience on response to host pheromone in Trichogramma sibericum (Hymenoptera: Trichogrammatidae). J. Insect Behav. 11:621–632.
Meiners, T. and Hilker, M. 1997. Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera, Chrysomelidae). Oecologia 112:87–93.
Meiners, T. and Hilker, M. 2000. Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol. 26:221–232.
Meiners, T., Wäckers, F., and Lewis, J. 2003. Associative learning of complex odors in parasitoid host location. The effect of molecule structure on the olfactory discrimination by the parasitoid Microplitis croceipes. Chem. Senses 28:231–236.
Meiners, T., Hacker, N., Anderson, P., and Hilker, M. 2005. Response of the elm leaf beetle to host plants induced by oviposition and feeding: the infestation rate matters. Entomol. Exp. Appl. 115:171–177.
Minardi, P. 1995. Cellular recognition in plant–bacteria interactions: biological and molecular aspects. Riv. Patol. Veg. 5:9–34.
Morris, C. E., Nicot, P. C., and Nguyen-The, C. 1996. Aerial Plant Surface Microbiology. Plenum Press, New York.
Müller, C. and Riederer, M. 2005. Plant surface properties in chemical ecology. J. Chem. Ecol. 31:3621–3651.
Mumm, R. and Hilker, M. 2005. The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem. Senses 30:1–7.
Mumm, R., Schrank, K., Wegener, R., Schulz, S., and Hilker, M. 2003. Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. J. Chem. Ecol. 29:1235–1252.
Nordlund, D. A. 1994. Habitat location by Trichogramma, pp. 155–164, in E. Waijnberg and S. A. Hassan (eds.). Biological Control with Egg Parasitoids. CAB International, Oxon.
Oliver, J. E., Doss, R. P., Williamson, R. T., Carney, J. R., and De Vilbiss, E. D. 2000. Bruchins-mitogenic 3-(hydroxy-propanoyl) esters of long chain diols from weevils of the Bruchidae. Tetrahedron 56:7633–7641.
Prokopy, R. J. and Roitberg, B. D. 2001. Joining and avoidance behavior in non-social insects. Annu. Rev. Entomol. 41:631–665.
Reddy, G. V. P., Holopainen, J. K., and Guerrrero, A. 2002. Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J. Chem. Ecol. 28:131–143.
Rohfritsch, O. 1992. Patterns in gall development, pp. 87–101, in J. D. Shorthouse and O. Rohfritsch (eds.). Biology of Insect-Induced Galls. Oxford University Press, Oxford.
Romeis, J., Shanower, T. G., and Zebitz, C. P. W. 1997. Volatile plant infochemicals mediate plant preference of Trichogramma chilonis. J. Chem. Ecol. 23:2455–2465.
Romeis, J., Shanower, T. G., and Zebitz, C. P. W. 1998. Physical and chemical plant character inhibiting the searching behaviour of Trichogramma chilonis. Entomol. Exp. Appl. 87:275–284.
Romeis, J., Babendreier, D., Wäckers, F. L., and Shanower, G. 2005. Habitat and plant specificity of Trichogramma egg parasitoids - Underlying mechanisms and implications. Basic Appl. Ecol. 3:215–236.
Rosetto, M., Belardinelli, M., Fausto, A. M., Marchini, D., Bongiorno, G., Maroli, M., and Mazzini, M. 2003. A mammalian-like lipase gene is expressed in the female reproductive accessory glands of the sand fly Phlebotomus papatasi (Diptera, Psychodidae). Insect Mol. Biol. 12:501–508.
Ruther, J., Reinecke, A., Thiemann, K., Tolasch, T., and Hilker, M. 2000. Mate finding in the forest cockchafer, Melolontha hippocastani, mediated by volatiles from plants and females. Physiol. Entomol. 25:172–179.
Schaller, F. and Weiler, E. W. 2002. Wound- and mechanical signalling, pp. 20–44, in D. Scheel and C. Wasternack (eds.). Plant Signal Transduction. Oxford University Press, Oxford.
Schroeder, R., Forstreuther, M., and Hilker, M. 2005. A plant notices insect egg deposition and changes its rate of photosynthesis. Plant Physiol. 138:470–477.
Seino, Y., Suzuki, Y., and Sogawa, K. 1996. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (Horvath) (Homoptera: Delphacidae). Appl. Entomol. Zool. 31:467–473.
Shapiro, A. M. and DeVay, J. E. 1987. Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera: Pieridae). Oecologia 71:631–632.
Shiojiri, K. and Takabayashi, J. 2003. Effect of specialist parasitoids on oviposition preference of phytophagous insects: encounter-dilution effects in a tritrophic interaction. Ecol. Entomol. 28:573–578.
Shorthouse, J. D. and Rohfritsch, O. 1992. Biology of Insect-Induced Galls. Oxford University Press, Oxford.
Smith, B. H. 1998. Analysis of interaction in binary mixtures. Physiol. Behav. 65:397–407.
Spiteller, D., Dettner, K., and Boland, W. 2000. Gut bacteria may be involved in interactions between plants, herbivores and their predators: microbial biosynthesis of N-acylglutamine surfactants as elicitors of plant volatiles. Biol. Chem. 381:755–762.
Steidle, J. L. M. and Van Loon, J. J. A. 2002a. Chemoecology of parasitoid and predator oviposition behaviour, pp. 291–348, in M. Hilker and T. Meiners (eds.). Chemoecology of Insect Eggs and Egg Deposition. Blackwell, Berlin.
Steidle, J. L. M. and Van Loon, J. J. A. 2002b. Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol. Exp. Appl. 108:133–148.
Steidle, J. L. M., Fischer, A., and Gantert, C. 2005. Do grains whisper for help? Evidence for herbivore-induced synomones in granary weevil infested wheat grains. Entomol. Exp. Appl. 115:239–245.
Suzuki, Y., Sogawa, K., and Seino, Y. 1996. Ovicidal reaction of rice plants against the whitebacked planthopper, Sogatella furcifera Horvath (Homoptera: Delphacidae). Appl. Entomol. Zool. 31:111–118.
Tooker, J. F. and De Moraes, C. M. 2005. Jasmonate in lepidopteran eggs and neonates. J. Chem. Ecol. 31:2753–2759.
Truitt, C. L., Wei, H. X., and Paré, P. W. 2004. A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 16:523–532.
Tumlinson, J. H. and Lait, C. G. 2005. Biosynthesis of fatty acid amide elicitors of plant volatiles by insect herbivores. Arch. Insect Biochem. Physiol. 58:54–68.
Tumlinson, J. H., Turlings, T. C. J., and Lewis, W. J. 1993. Semiochemically mediated foraging behavior in beneficial parasitic insects. Arch. Insect Biochm. Physiol. 22:385–391.
Turlings, T. C. J. and Wäckers, F. 2004. Recruitment of predators and parasitoids by herbivore injured plants, pp. 21–75, in R. T. Cardé and J. G. Millar (eds.). Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge.
Underwood N., Anderson K., and Inouye B. D. 2005. Induced vs. constitutive resistance and the spatial distribution of insect herbivores among plants. Ecology 26:594–602.
Varma, A., Abbott, L., Werner, D., and Hampp, R. 2004. Plant Surface Microbiology. Springer, Berlin Heidelberg New York.
Vet, L. E. M. and Dicke, M. 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37:141–172.
Wegener, R., Schulz, S., Meiners, T., Hadwich, K., and Hilker, M. 2001. Analysis of volatiles induced by oviposition of a phytophagous insect. J. Chem. Ecol. 27:499–515.
Yamasaki, M., Yoshimura, A., and Yasui, H. 2003. Genetic basic of ovicidal response to whitebacked planthopper (Sogatella furcifera Horvath) in rice (Oryza sativa L.). Mol. Breed. 12:133–143.
Zimmermann, S., Ehrhardt, T., Plesch, G., and Mueller-Roeber, B. 1999. Ion channels in plant signaling. Cell. Mol. Life Sci. 55:183–203.
Zuk, M. and Kolluru, G. R. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73:415–438.
Zweigelt, F. 1931. Blattlausgallen. Histologische und biologische Studien an Tetraneura- und Schizoneuragallen. Die Blattlausgallen im Dienste prinzipieller Gallenforschung. Monogr. Angew. Entomol. (Suppl. Z. Angew. Entomol. 27) 11:1–684.
Acknowledgments
We thank our students Melanie Thiers and Daniel Kämmer for helping us in providing the photo material shown in Fig. 1. We are also grateful for the interesting questions raised by two anonymous reviewers on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hilker, M., Meiners, T. Early Herbivore Alert: Insect Eggs Induce Plant Defense. J Chem Ecol 32, 1379–1397 (2006). https://doi.org/10.1007/s10886-006-9057-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9057-4