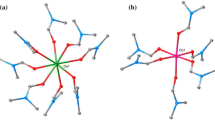
The reaction of the polyoxomolybdate anion \([\hbox{Mo}_{32}^{\rm VI}\hbox{(MoNO)}_{4}\hbox{O}_{108}(\hbox{H}_{2}\hbox{O})_{16}]^{12-} =\{\hbox{Mo}_{36}\hbox{(NO)}_{4}\}\) with FeII salts and hydrazine sulfate yields the cluster anion \([\hbox{Mo}^{\rm V/VI}_{51}(\hbox{Mo}^{\rm VI}\hbox{O})_{2}\hbox{Fe}^{\rm III}_{6}\hbox{(MoNO)}_{6}\hbox{O}_{176}\hbox{(OH)}_{3} (\hbox{H}_{2}\hbox{O})_{22}]^{15-}\) (1a), isolated as its corresponding ammonium salt (NH4)15 1a · 36H2O (1) (space group P63/mmc, a=b=23.607(5) Å, c=26.767(6) Å, 4076 unique reflections, 293 parameters). Despite the absence of oxygen and the reducing conditions the product comprises only FeIII centers as determined by a combination of spectroscopic, magnetic and crystallographic analysis. Reduction of peripheral Mo centers causes the aggregation of two additional [MoVIO]4+ groups onto the archetypal {Mo57M6} structure. The single-crystal X-ray determined structure of 1 is reported.




Similar content being viewed by others
References
Hill C. L. (1998). Chem. Rev. 98: 1 (special issue on polyoxometalates)
Müller A., Kögerler P. and Dress A. W. M. (2001). Coord. Chem. Rev. 222: 193
A. Müller, P. Kögerler, and C. Kuhlmann (1999). Chem. Commun. 1347
D.-L. Long, P. Kögerler, L. J. Farrugia, and L. Cronin (2003). Angew. Chem. Int. Ed. 42, 4180; D.-L. Long, P. Kögerler, L. J. Farrugia, and L. Cronin (2005). Dalton Trans. 1372
Krebs B., Stiller S., Tytko K. H. and Mehmke J. (1991). Eur. J. Solid Stat. Inorg. Chem. 28: 883
Müller A., Krickemeyer E., Dillinger S., Bögge H., Plass W., Proust A., Dloczik L., Menke C., Meyer J. and Rohlfing R. (1994). Z. Anorg. Allg. Chem. 620: 599
Gatteschi D., Sessoli R., Plass W., Müller A., Krickemeyer E., Meyer J., Sölter D. and Adler P. (1996). Inorg. Chem. 35: 1926
(a) A. Müller, W. Plass, E. Krickemeyer, S. Dillinger, H. Bögge, A. Armatage, A. Proust, C. Beugholt, and U. Bergmann (1994). Angew. Chem., Int. Ed. Engl. 33, 849; (b) A. Müller, H. Bögge, E. Krickemeyer, and S. Dillinger (1994). Bull. Pol. Acad. Sci. Chem. 42, 291.
Lutz H. D., Nagel R., Mason S. A., Müller A., Bögge H. and Krickemeyer E.(2002). J. Solid State Chem. 165: 199
Müller A., Plass W., Krickemeyer E., Sessoli R., Gatteschi D., Meyer J., Bögge H., Kröckel M. and Trautwein A. X. (1998). Inorg. Chim. Acta 271: 9
Kögerler P. and Müller A. (2003). J. Appl. Phys. 93: 7101
Müller A., Meyer J., Krickemeyer E., Beugholt C., Bögge H., Peters F., Schmidtmann M., Kögerler P. and Koop M. J. (1998). Chem. Eur. J. 4: 1000
Nicoara A., Patrut A., Margineanu D. and Müller A. (2003). Electrochem. Commun. 5: 511
Sheldrick G. M. (1997). SHELXTL Version 5.1. Bruker Analytical X-Ray Systems, Inc., Madison, WI
Farrugia L. J. (1999). J. Appl. Crystallogr. 32: 837
Blessing R. H. (1995). Acta Crystallogr A51: 33
Acknowledgements
Ames Laboratory is operated for the U.S. Department of Energy by Iowa State University under Contract No. W-7405-Eng-82. Y.L.M. thanks MRDA/CRDF for a travel fellowship (award no. MTFP-04–03). We thank Professor B. H. Huynh for the 57Fe Mössbauer data.
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to Professor Michael T. Pope.
Rights and permissions
About this article
Cite this article
Fielden, J., Malaestean, Y.L., Ellern, A. et al. Inducing Molecular Growth in an {Mo57Fe6}-type Nanocluster: Synthesis, Structure, and Properties of \(\{\hbox{Mo}_{57}\hbox{(Mo)}_{2}\hbox{Fe}^{\rm III}_{6}\}\)*. J Clust Sci 17, 291–302 (2006). https://doi.org/10.1007/s10876-006-0053-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-006-0053-1