Abstract
Introduction
Changes in the levels of serum cytokines and growth factors are associated with response to therapy. We examined cytokine, chemokine, and growth factor levels in serum collected from normal volunteers or metastatic melanoma patients receiving dendritic cell-based immunotherapy.
Materials and Methods
Using an array for 42 cytokines, chemokines, or growth factors, sera from normal controls and metastatic melanoma patients at baseline and week 4 were analyzed for qualitative changes. Quantitative determination of the levels of the chemokine thymus and activation-regulated chemokine (TARC/CCL17) was determined by enzyme-linked immunosorbent assay (ELISA).
Results
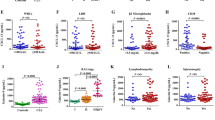
Significant qualitative differences were noted in serum cytokine, chemokine, and growth factor levels of metastatic melanoma patients versus the normal controls at baseline. The results also demonstrated a significant decrease in the level of angiogenin (P = 0.026) and a significant increase in TARC/CCLl7 (P = 0.008) from week 0 to week 4 which was associated with improved overall survival (P = 0.059). Higher TARC/CCL17 levels were observed by ELISA at week 4 and a log-rank comparison revealed a significant association between high serum TARC/CCL17 levels at week 4 and progression-free survival (P = 0.005). Receiver–operator characteristic analysis revealed that week 4 serum TARC/CCL17 levels were predictive of progression-free and overall survival, indicating that serum TARC/CCL17 might be of predictive value of response to dendritic cell-based anti-melanoma immunotherapy.




Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi:10.3322/CA.2007.0010.
Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–203. doi:10.1172/JCI31205.
Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–50. doi:10.1111/j.1600-065X.2007.00575.x.
Aarntzen EH, Figdor CG, Adema GJ, Punt CJ, de Vries IJ. Dendritic cell vaccination and immune monitoring. Cancer Immunol Immunother. 2008;57:1559–68. doi:10.1007/s00262-008-0553-y.
Feili-Hariri M, Falkner DH, Morel PA. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J Leukoc Biol. 2005;78:656–64. doi:10.1189/jlb.1104631.
Vissers JL, Hartgers FC, Lindhout E, Teunissen MB, Figdor CG, Adema GJ. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2001;69:785–93.
Nagorsen D, Marincola FM, Panelli MC. Cytokine and chemokine expression profiles of maturing dendritic cells using multiprotein platform arrays. Cytokine. 2004;25:31–5. doi:10.1016/j.cyto.2003.08.012.
Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–83.
Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515–22. doi:10.1097/00008390-200512000-00006.
Vihinen P, Kallioinen M, Vuoristo MS, Ivaska J, Syrjanen KJ, Hahka-Kemppinen M, et al. Serum angiogenin levels predict treatment response in patients with stage IV melanoma. Clin Exp Metastasis. 2007;24:567–74. doi:10.1007/s10585-007-9093-7.
Grimm EA, Smid CM, Lee JJ, Tseng CH, Eton O, Buzaid AC. Unexpected cytokines in serum of malignant melanoma patients during sequential biochemotherapy. Clin Cancer Res. 2000;6:3895–903.
Choi D, Perrin M, Hoffmann S, Chang AE, Ratanatharathorn V, Uberti J, et al. Dendritic cell-based vaccines in the setting of peripheral blood stem cell transplantation: CD34 + cell-depleted mobilized peripheral blood can serve as a source of potent dendritic cells. Clin Cancer Res. 1998;4:2709–16.
Luft T, Pang KC, Thomas E, Bradley CJ, Savoia H, Trapani J, et al. A serum-free culture model for studying the differentiation of human dendritic cells from adult CD34+ progenitor cells. Exp Hematol. 1998;26:489–500.
Dillman RO, Nayak SK, Beutel L. Establishing in vitro cultures of autologous tumor cells for use in active specific immunotherapy. J Immunother Emphasis Tumor Immunol. 1993;14:65–9.
Dillman RO, Beutel LD, Cornforth AN, Nayak SK. Short-term tumor cell lines from renal cell carcinoma for use as autologous tumor cell vaccines in the treatment of kidney cancer. Cancer Biother Radiopharm. 2000;15:161–8. doi:10.1089/cbr.2000.15.161.
Selvan SR, Dillman RO, Fowler AW, Carbonell DJ, Ravindranath MH. Monitoring response to treatment in melanoma patients: potential of a serum glycomic marker. Int J Cancer. 2008;122(6):1374–83.
Dillman RO, Beutel LD, Barth NM, de Leon C, O’Connor AA, DePriest C, et al. Irradiated cells from autologous tumor cell lines as patient-specific vaccine therapy in 125 patients with metastatic cancer: induction of delayed-type hypersensitivity to autologous tumor is associated with improved survival. Cancer Biother Radiopharm. 2002;17:51–66. doi:10.1089/10849780252824073.
Shapiro SS, Wilk M. An analysis of variance test for normality (complete samples). Biometrika. 1965;52:591–611.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36.
Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13:2422–8. doi:10.1158/1078-0432.CCR-06-1805.
Dillman RO, Selvan SR, Schiltz PM. Patient-specific dendritic-cell vaccines for metastatic melanoma. N Engl J Med. 2006;355:1179–81. doi:10.1056/NEJMc061667.
Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9. doi:10.1002/ijc.22024.
Guo Z, Zhang M, Tang H, Cao X. Fas signal links innate and adaptive immunity by promoting dendritic-cell secretion of CC and CXC chemokines. Blood. 2005;106:2033–41. doi:10.1182/blood-2004-12-4831.
Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, et al. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol. 2005;83:525–35. doi:10.1111/j.1440-1711.2005.01365.x.
de Vries IJ, Eggert AA, Scharenborg NM, Vissers JL, Lesterhuis WJ, Boerman OC, et al. Phenotypical and functional characterization of clinical grade dendritic cells. J Immunother. 2002;25:429–38.
Kanagawa N, Niwa M, Hatanaka Y, Tani Y, Nakagawa S, Fujita T, et al. CC-chemokine ligand 17 gene therapy induces tumor regression through augmentation of tumor-infiltrating immune cells in a murine model of preexisting CT26 colon carcinoma. Int J Cancer. 2007;121:2013–22. doi:10.1002/ijc.22908.
Okada N, Sasaki A, Niwa M, Okada Y, Hatanaka Y, Tani Y, et al. Tumor suppressive efficacy through augmentation of tumor-infiltrating immune cells by intratumoral injection of chemokine-expressing adenoviral vector. Cancer Gene Ther. 2006;13:393–405. doi:10.1038/sj.cgt.7700903.
Nakazaki Y, Hase H, Inoue H, Beppu Y, Meng XK, Sakaguchi G, et al. Serial analysis of gene expression in progressing and regressing mouse tumors implicates the involvement of RANTES and TARC in antitumor immune responses. Mol Ther. 2006;14:599–606. doi:10.1016/j.ymthe.2006.04.014.
Inoue H, Iga M, Xin M, Asahi S, Nakamura T, Kurita R, et al. TARC and RANTES enhance antitumor immunity induced by the GM-CSF-transduced tumor vaccine in a mouse tumor model. Cancer Immunol Immunother. 2008;57:1399–411. doi:10.1007/s00262-008-0476-7.
Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–8. doi:10.1093/intimm/11.1.81.
Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte–macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–46.
Acknowledgments
We would like to acknowledge Sarah Tillman and Andrea Beatty for their assistance in generating the tumor and dendritic cell lines, William McRorie for his assistance in the TARC/CCL17 ELISA, and Micheal Farr for his contribution to the cytoarray work. Acknowledgement should also go to Dr. Senthamil Selvan for his input in writing this manuscript. Funding for this work was provided by the Hoag Hospital Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cornforth, A.N., Lee, G.J., Fowler, A.W. et al. Increases in Serum TARC/CCL17 Levels Are Associated with Progression-Free Survival in Advanced Melanoma Patients in Response to Dendritic Cell-Based Immunotherapy. J Clin Immunol 29, 657–664 (2009). https://doi.org/10.1007/s10875-009-9299-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-009-9299-3