Abstract
The size-segregated chemical composition of aerosol particles was investigated during 1 year at the puy de Dôme (1,465 m a.s.l.), France. These measurements aimed to a better understanding of the influence of the air mass origin on the size-segregated chemical composition of the aerosol at an altitude site. Mountain site measurements are important because they are representative of long range transport and useful for model validation. PM1 mass concentration exhibits a seasonal variability with a summer maximum. The composition of PM1 did not change significantly in terms of relative contribution of water soluble inorganic ions but is rather variable in term of total mass concentrations. For the PM10-1, a different seasonal behaviour was found with maxima concentrations in autumn-winter. Aerosols were classified into four different categories according to their air mass origin: marine, marine modified, continental and Mediterranean. The PM10 aerosol mass at 50 % relative humidity was close to 2.5 μg m−3 in the marine, 4.3 μg m−3 in the marine modified, 10.3 μg m−3 in the continental and 7.7 μg m−3 in the Mediterranean sectors. We noted that the influence of the air mass origin (on the chemical properties) could be seen especially on the PM10-1. A significant PM10-1 mode was found in marine, modified marine, and Mediterranean air masses, and PM1 dominated in the continental air masses samples. As a result, the aerosol chemical composition variability at the puy de Dôme is a function of both the season and air mass type and we provide a chemical composition of the aerosol as a function of each of these environmental factors.
Similar content being viewed by others
1 Introduction
Information on the atmospheric composition, from the local to the global scale, is of strategic value for climate and air quality related studies. Despite important improvements of the Earth observing strategy in the last decade, there is still a lack of knowledge. Observations of the chemical composition of the atmosphere with temporal, horizontal and vertical resolution are crucially needed to 1) satisfy and verify current legislation, 2) to validate and help to improve our understanding of atmospheric processes, and 3) to allow accurate predictions of future atmospheric states by providing inputs to forecasting models.
A process-level understanding of the atmospheric cycle of key variables requires continuous observations with hourly to daily resolution. At present, this is only provided by ground-based instrumentation. Clearly, the ground-based component is an essential element of the observing system because long-term and high-quality observations are required for improving our understanding of atmospheric processes. The ground-based component, however, suffers from uneven global and regional coverage of atmospheric observations. In particular, while observations in the mixed layer are to some extent sufficient, at least in OECD countries (Organisation for Economic Co-operation and Development), there is a clear lack of information from the upper troposphere, i.e. derived from stations located at high altitude (Laj et al. 2009). Temporal variations in the upper troposphere of aerosol properties, especially of non-regulated advanced parameters such as chemical composition, is required for investigating climate, environmental, and health effects in the context of future global changes (Andrews et al. 2011).
Monitoring of the chemical composition of aerosol particles has been performed at many locations within the mixed layer (e.g. Mészáros et al. 1997; Heintzenberg et al. 1998; Lestari et al. 2003; Jaffe et al. 2005; Saliba et al. 2007; Putaud et al. 2010 and many others). However, long term measurement series are scarce at high altitude sites. Kasper and Puxbaum (1998) monitored sulphate, nitrate and ammonium at Sonnblick (3,106 m a.s.l.) during 2 y and showed marked seasonal cycles with low concentrations in winter and high concentrations in summer. Cozic et al. (2008) studied organic and inorganic compounds for 7 y at the Jungfraujoch station (3,580 m a.s.l.) and showed that the maximum of total suspended particles (TSP) mass corresponds to periods with highest temperatures, but they did not find any year to year trend. The EU-CARBOSOL project (Present and Retrospective State of Organic versus Inorganic Aerosol over Europe: Implications for Climate; Legrand and Puxbaum 2007) provided very important results concerning the variability of aerosol chemistry at various high altitude sites across Europe. They observed that organic matter concentrations were as high as the ones of total inorganic soluble compounds and that both had a seasonal behaviour with a summer maximum.
The present paper aims at presenting results by specifically focusing i) on the variability of aerosol composition, ii) on the size-dependant chemistry, and iii) on the link between meteorological factors (air mass origin) and chemical composition at a mountain site representative of a large footprint area (Asmi et al. 2011). Therefore, this paper reports aerosol size-segregated chemistry and investigates the source origin of the aerosol components.
2 Experimental
2.1 Sampling site and method
Measurements were conducted at the altitude site puy de Dôme (pdD) summit (1,465 m a.s.l., 45° 46′ 20″ N, 2° 57′ 57″ E) . A detailed description of the sampling site can be found in a number of previous studies (Wobrock et al. 2001; Venzac et al. 2009). The station is located in a natural background area, restricted during winter, authorized to cars during the spring and autumn periods, and only to organized buses during the summer period. Previous analysis showed limited impact of emissions from both the city of Clermont-Ferrand and local traffic on chemical measurements at the site (Sellegri et al. 2003). Therefore, this site can be classified as a rural background site according to Putaud et al. (2004) and high altitude site (z > 1,000 m a.s.l.) according to Asmi et al. (2011). According to modelling results (Fig. 1) and LIDAR results (Boulon et al. 2011), the pdD often lies in the mixed layer during the day and in the residual layer during the night during summer. During the winter, the station is located above the mixed layer.
Meteorological parameters (temperature, wind speed and direction, and relative humidity - RH) are monitored permanently since October 1995, showing temperatures varying between −20 and 10°C during winter and between 5 and 20°C during summer. The site was often used for investigations of cloud chemistry and aerosol-cloud interactions (Sellegri et al. 2003; Marinoni et al. 2004), due to the frequent formation of clouds (more than 50 % of the time between November and March).
Aerosol sampling inlets (1 m above the station roof, i.e. >2.5 m a.g.l.) were designed to sample during clear sky conditions as well as during cloudy conditions using a whole air inlet (WAI; Sellegri 2002) which ensured efficient sampling of both cloud droplets residues and interstitial aerosol in the presence of clouds. Wind speed was decreased in the vicinity of the WAI by a series of metallic fences ensuring efficient sampling even at elevated wind speed. Based upon theoretical considerations, the WAI is capable of efficiently (50 % efficiency) sampling droplets <35 μm for wind speed <10 m s−1. During cloudy conditions, interstitial aerosols and cloud droplets residues were sampled simultaneously at a RH close to 50 % by mean of heating coil.
Size segregated sampling was performed for a full year (April 2006–April 2007) with a 30 lpm 13-stages low pressure cascade impactor (Dekati type) with cut-off aerodynamic diameters of 0.03, 0.06, 0.10, 0.17, 0.26, 0.40, 0.65, 1.02, 1.65, 2.51, 4.07, 6.73 and 10.42 μm. The different modes have been calculated by summing the impactor’s stages. The PM0.1 mode is constituted by particles with a diameter comprised between 30 nm and 105 nm, the PM1-0.2 mode is composed by particles with a diameter comprised between 105 nm and 1.02 μm and the PM10-1 mode by particles which diameter is comprised between 1.02 μm and 10.42 μm. Sampling time integration varied between 3 and 4 d and was performed on a weekly basis for a full year. The collection plates were custom-made out of aluminium foil and analysed for ionic species. Field blank were collected on a weekly basis.
2.2 Chemical analysis
Collection plates from the impactor were handled and extracted under a class 100 laminar flow hood. Before sampling, the aluminium foil were washed with MeOH and rinsed with Milli-Q water. Then, the collection plates were dried in a class 1,000 clean room (T = 22°C ± 1; ΔP = + 60 Pa; RH = 50 % ± 10; Clermont-Ferrand, France). Before and after sampling, the collection plates were weighed, using a microbalance UMT2 Mettler Toledo, after 24 h in the clean room in order to reach the equilibrium temperature and RH. After weighing, the collection plates were analyzed with the chromatographic method described below.
The water soluble inorganic ions (WSII) were analyzed by ion chromatography using the methodology described by Ricard et al. (2002). Impaction plates were extracted in their storage bottle for about 10 min using 10 ml of Milli-Q water and manual agitation. Extract were filtrated with the filter caps which have 20 μm porosity. Cations (sodium, ammonium, potassium, magnesium, and calcium) were analysed with a Dionex ICS-1500 chromatograph, using a CS16 column, a CG16 guard column and chemical regeneration was made with a CSRS ULTRA II autosuppressor and a 0.20 % MSA eluent. Concentrations of major water soluble anions (chloride, nitrate, sulphate and oxalate) were determined with a Dionex IC25 chromatograph, using an AS11 column, an AG11 guard column and an ASRS ULTRA II autosuppressor. Injection was performed using a KOH gradient and an EG40 eluent generator. Anions and cations were injected in parallel with an AS40 automated sampler with an injection loop of 750 μL.
The measurement of NO -3 is problematic and may be affected by large uncertainties (Chang et al. 2001). On the one hand, there is the possibility of a positive artefact by the adsorption of gaseous HNO3 onto the filter material, which will be then accounted as NO -3 (Ten Brink et al. 2009). However, Weber et al. (2003) found that this may not be a significant artefact. On the other hand, NO -3 can be evaporated in the form of semi volatile NH4NO3. It is worthy to note that there is some NO -3 losses during impactor sampling due to low pressures in the instrument, but they are generally lower than those observed with filter samplers (Zhang and McMurry 1987, 1992; Wang and John 1988). For impactor sampling, Wang and John (1988) determined wall losses of NO -3 of 1.1 % for particles larger than 2 μm, of 3.0 % for particles between 0.5 and 2 μm and of 8.1 % for particles smaller than 0.5 μm. They also estimated evaporation losses up to 7 %, in agreement with Wall et al. (1988). Concerning the storage before weighing, Dougle and Ten Brink (1996) found no significant loss of NO -3 at 0°C and 20°C over a period of several days and a small loss of weigh (within 10 %) at room temperature during 2 months. In the following, NO -3 data will be corrected only from the field blank.
The arithmetic averages of concentrations for the field blanks were subtracted from sample concentrations. The contribution of field blanks varied according to the chemical species, the highest contribution was observed for the compounds which had the smallest concentrations such as Cl- and Na+ was 3.6 and 4.6 % respectively. When concentrations are lower than the detection limit (see Table 1), the level was set at the detection limit for the calculation of the means, which are therefore upper limits. Atmospheric concentrations sampled at the atmospheric pressure of the pdD (around 850 hPa) were normalized to the standard conditions of pressure (1,013 hPa).
3 Results
3.1 Time variation of the weighed mass
The average monthly mass (Fig. 2) is calculated by averaging the 3 or 4 weekly values obtained with the sum of the weighed masses of all impactor stages of a given run. The PM10 mass is calculated with the sum of the weighed mass from stage 1 to stage 12 and the PM1 is the sum of the weighed masses of stages 1 to 7, the concentration values are given in Table 2.
We observe a maximum during summer and a minimum during winter, which is slightly different from what has been observed in the mixed layer in Southern Europe. Bergametti et al. (1989) and Querol et al. (1998) showed a maximum in spring and a minimum in autumn. This behaviour was not observed everywhere in mixing layer stations. In some places, a maximum was observed during winter and a minimum during summer, presumably due to a larger impact of combustion sources during winter and the “trapping” of these particles in a thinner mixed layer (Mészáros et al. 1997; Röösli et al. 2001; Calvo et al. 2008; Vecchi et al. 2009). At high altitude stations such as the Jungfraujoch (3,454 m a.s.l.), Switzerland (Baltensperger et al. 1991) or in Manali (2,050 m a.s.l.), India (Gajananda et al. 2005), a summer maximum in aerosol mass was usually observed.
The PM1 concentration is higher than observed at Jungfraujoch (1.7 μg m−3 and 2.5 μg m−3 in winter and summer respectively, Cozic et al. 2008). We observe a maximum in July and a minimum in January for the sub-micron particles, as for the PM10 mass. The PM10-1 concentration is of the same order of magnitude than at the Jungfraujoch (2.4 μg m−3 and 2.0 μg m−3 in winter and summer respectively, Cozic et al. 2008) with little seasonality. Hence, the seasonal behaviour of the PM10 concentrations is mainly driven by the PM1 concentration seasonal behaviour.
In fact, the PM1 fraction represents the dominant fraction of the PM10 at pdD (64 ± 12 %), as opposed to sites closer to natural primary emissions sources (Van Dingenen et al. 2004). This is particularly the case in spring-summer, when the PM1/PM10 reaches about 80 %. This seasonal behaviour is similar to those observed on particle number concentrations, with summer particle number concentrations being 4 times higher than winter particle number concentrations (Venzac et al. 2009). Venzac et al. (2009) noticed the importance of the atmospheric vertical dynamics on the seasonal variability of the aerosol number concentration. This feature could also be partly attributed to higher photochemical reactions (Lee et al. 2001) and higher particles formation caused by higher solar radiation (Birmili and Wiedensohler 2000) in summer, as the observed actinic flux experiences a strong maximum in summer.
3.2 WSII time series
The concentrations are given in Table 3. The PM1 WSII presents a minimum in winter and a maximum in summer. As for the PM1, the PM10 WSII mass is higher in summer and lower in winter. Again, the seasonal variation of WSII follows an opposite seasonal trend at mixed layer stations in Hungary (Mészáros et al. 1997), but a similar trend at high altitude sites such as the Sonnblick (3,106 m a.s.l.), Austria (Kasper and Puxbaum 1998), at the Jungfraujoch (3,580 m a.s.l.), Switzerland (Cozic et al. 2008) and at the Schauinsland (1,205 m a.s.l.), Germany (Salvador et al. 2010). The PM10 WSII winter concentration is lower than those found by Sellegri et al. (2003) at pdD during the winters 2000 and 2001. Within the CARBOSOL project, Pio et al. (2007) measured PM10 WSII average concentrations at pdD station of 3.7 μg m−3 in summer and 2.2 μg m−3 in winter, comparable to the WSII that we report.
In the following section, we will analyze the aerosol composition in more details in order to further understand what drives the seasonal variability of the chemical composition (Fig. 3).
3.3 Detailed chemical compositions
3.3.1 PM1
Nitrate concentrations show a minimum in autumn and a maximum in spring. Nitrate is derived from precursors (e.g. HNO3, N2O5) emitted by anthropogenic sources and can condense on the surface of particles (Tolocka et al. 2004; Anlauf et al. 2006). In the fine fraction, it is linked with ammonia (Putaud et al. 2004). Such spring maximum has been attributed to mountain breeze at Montseny (Pey et al. 2010), although the configuration of the pdD mountain chain does not favour the formation of mountain breezes. It could also be attributed to regional pollution episodes (Pérez et al. 2008). Differently, for SO 2-4 and NH +4 , the minimum is observed in winter and the maximum is observed in summer, which is in agreement with the PM10 mass seasonal variation, linked to the seasonal variation of the mixed layer height. The high levels of SO 2-4 during summer have been described by Rodriguez et al. (2002), they pointed out the importance of photochemical processes.
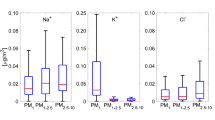
We calculated the ratio between analysed cations and anions following the season. As shown by Fig. 4, the cation-to-anion ratio is smaller for particles with an aerodynamic diameter comprised between 0.2 μm and 1 μm and increasing when going to either larger or smaller particle sizes. This feature is the same of the one observed at remote and rural sites in Northern Europe (Kerminen et al. 2001). Except in summer, the averaged cation-to-anion ratio is close to unity. During summer, there was a large amount of sulphate, making our ionic balance not satisfied and these aerosol particles quite acidic.
The mean NO -3 /SO 2-4 ratio is 0.3, which is similar to what was observed at Monte Cimone, high altitude site (2,165 m a.s.l.; Marenco et al. 2006). We observe a high seasonal variability of the mass ratio between NO -3 /SO 2-4 ; it is higher during winter and spring (0.1 and 0.9, respectively) and lower during summer and autumn (0.03 for both).
We find an ubiquitous significant fraction of undetermined mass. The influence of water present on impaction foil is estimated assuming a particle hygroscopic growth factor at 50 % RH. Most of hygroscopic particles (sea salt, ammonium sulphate, ammonium nitrate) show growth factors equal to 1 at 50 % RH (Hansson et al. 1998; Krämer et al. 2000; Cruz and Pandis 2000; Hämeri et al. 2000; Mikhailov et al. 2004). Carbonaceous particles also have a hygroscopic growth factor of 1 (Weingartner et al. 1997). In addition to that, Putaud et al. (2004) showed that gravimetric measurements at 50 % RH led to PM10 and PM2.5, respectively, 1.09 and 1.07 times as high as gravimetric measurements at 20 % RH and water should not contribute to the weighed mass at 50 % RH. Moreover, the main metal oxides concentrations previously detected by PIXE in pdD samples in the past were Al2O3, SiO3, and FeO, and very little contribution from other metal oxides were found. Altogether, the contribution of these three metal oxides of crustal origin were always less than 4 % of the total mass of the aerosol, except in strong Saharan dust events in which it reached 26 % of the PM10-1 mode mass (Sellegri et al. 2003). Thus, based on the results from Sellegri et al. (2003) who achieved a mass balance closure within 15 % on cascade impactor samples, we tentatively attribute the undetermined mass (weighed but not analyzed) to EC + OM in the PM1. In favour of the hypothesis, we found that the oxalate concentration correlates significantly with the undetermined mass with R2 comprised 0.88 and 0.99. The concentration of the undetermined mass is higher during the summer and lower in winter. A spring-summer maximum and the autumn-winter minimum have been observed for OM as well at Schauinsland, Germany (1,205 m a.s.l.) and at Sonnblick, Autria (3,106 m a.s.l.) (Pio et al. 2007) with summer concentrations being 2 to 3 times higher than the winter concentrations. We calculate concentrations lower than those reported for CARBOSOL at pdD (Pio et al. 2007), possibly due to year to year variability but also expected because of different cut-off (PM10 for CARBOSOL).
The composition of the PM0.1 range has been the focus of several studies, as it represents the most numerous fraction of particles acting as CCN (Raga and Jonas 1993; Martin et al. 1994; Khlystov et al. 1997, 1998). The PM0.1 has a chemical composition dominated by undetermined mass (Table 4). The SO 2-4 is the predominant WSII compounds, the NH +4 and NO -3 contributions being low. Even if the proportions of each compound are slightly different between the PM1 and PM0.1, the same compounds are predominant in both modes.
We notice that the behaviour of the PM1 is mainly driven by the undetermined mass and then by SO 2-4 , NO -3 and NH +4 , in agreement with what is usually observed at altitude stations and mixed layer sites. We do not observe a strong seasonality in the relative composition of the PM1. This observation favours the hypothesis of the mixed layer dynamics being the main parameter driving the aerosol concentrations at the site.
3.3.2 PM10-1
Nitrate is a compound which can be found both in the PM1 and in the PM10-1 (Putaud et al. 2004). A clear seasonal pattern is observed with a higher contribution of the PM10-1 NO -3 to the PM10 NO -3 in the autumn-winter period (Fig. 5) even if the concentration of PM10-1 NO -3 is higher in autumn but lower in winter. The autumn maximum could be attributed to the high number of samples originating from Mediterranean region (see later, section III.5.1). In the PM10-1, it is bound with alkaline sea salt and/or mineral dust particles (Harrison and Kitto 1990; Pakkanen et al. 1996; Hanke et al. 2003; Krueger et al. 2004; Sullivan et al. 2007). The reaction of HNO3 to sea salt has been pointed out in several publications (Robbins et al. 1959; Martens et al. 1973; Harrison and Pio 1983; Pakkanen et al. 1996; Plate and Schulz 1997; Vignati et al. 2001; Sørensen et al. 2005), resulting in a loss of Cl- relative to the Cl-/Na+ ratio in the sea composition. The minimum of PM10-1 NO -3 in winter is consistent with the fact that there is no loss of Cl- during this season (NaCl molar ratio of 1.05, which is very close to what is described for sea water i.e. 1.2 (Wilson 1975)).
The PM10-1 SO 2-4 exhibits a spring maximum and a winter minimum. The mean NO -3 /SO 2-4 ratio is 2, which is higher than what was observed for rural sites in Spain, between 0.4 and 0.7 (Rodriguez et al. 2004; Viana et al. 2008; Pey et al. 2009) and higher than the one we observed for PM1.
The NH +4 maximum is in spring and the minimum in winter.
The winter sea salt (Na+ + Cl-) average concentration is twice as high as the ones found by Sellegri et al. (2003) at the pdD during winter. The average sea salt concentrations of every seasons at pdD are 10 times higher than the ones measured (for PM10) by Cozic et al. (2008), which is expected as the PdD is farther west compared to the Jungfraujoch. We observe that sea salt has a higher concentration in winter and autumn. A sea salt mass fraction maximum in winter was already observed by Jaffe et al. (2005) for sites located inland from the coast by 200 km, who attributed this maximum to higher wind speed during this season. Indeed, Yan et al. (2002) observed highest wind speed during winter and lowest wind speed during summer over the Atlantic Ocean.
As previously discussed, we find a significant fraction of undetermined mass. Since the undetermined mass correlates significantly with C2O 2-4 in spring (R2 = 0.85) and in winter, summer and autumn (R2 = 0.5) and with nssCa2+ (0.3 < R2 < 0.7), we hypothesize this fraction to be mostly composed by OM and minerals. The undetermined mass mean concentration does not present specific seasonal behaviour. It is largely predominant since it represents 58 to 71 % of the PM10-1.
3.3.3 Size
Size distributions (Fig. 3) globally show PM1 with a modal diameter at 0.4 μm, which is also the same for each individual major water soluble inorganic compound (NO -3 , SO 2-4 and NH +4 ), and PM10-1 with a modal diameter at 1.6 μm. We observe that the mass ratio between PM1/PM10-1 is highly variable according to the season, together with the composition of the PM10-1.
Moreover, we observe that the intra-seasonal variability of the aerosol is higher than the inter-seasonal variability, for both PM1 and PM10-1. Within a single season, we still need to establish which factor is determining aerosol concentration and composition. We will in the following section focus on the effect of air mass type on the aerosol composition at pdD.
4 Average chemical composition in various air mass types
4.1 Classification of air masses
The goal of this classification is to identify air masses with a specific chemical signature. To establish this classification, 5 d back-trajectories were calculated using the HYSPLIT transport and dispersion model and/or READY website (http://www.arl.noaa.gov/ready.html) (Draxler and Rolph 2003; Rolph 2003) for the sampling period of the impactor (ca. 3 d on average). The back-trajectories were calculated at 00:00 UTC and 12:00 UTC and their end point was centered at the pdD (45°46′20″N, 2°57′57″E, 1,465 m a.s.l.). The air masses were classified following their origin (Fig. 6) if they were homogeneous (considering the geographical origin within the concerned sector, not the meteorological conditions during transport or sampling) during the three calculation days. If a change of air mass type occurred, they were classified as undefined.
These sectors were selected depending on the main aerosols sources. Marine air masses originated from the Atlantic Ocean and arrived at pdD from the west coast of France. Marine-modified air masses also originated from the Atlantic Ocean, but crossing a populated area of North-Western Europe (England, Denmark, etc.) before reaching France and then pdD. Continental air masses originated from areas located to the east of the site. Mediterranean air masses originated from south of the site, often from North Africa or the Mediterranean Sea.
We report the number of samples performed in each air mass type according to the season (DJF for winter, MAM for spring, JJA for summer and SON for autumn) (Fig. 7). We can notice that air mass types are not evenly spread over the seasons, which might have influenced the seasonal variability observed in the previous sections. The Mediterranean sector comprises most of the air mass types sampled during autumn, which might have an impact on the aerosol mean composition during this season in our data set. The mass concentrations are given in Table 5.
4.2 PM1
The highest weighed mass on cascade impactor samples is measured for the continental samples, then for the mediterranean samples and last for the marine modified and marine samples.
Concerning concentrations, the major WSII and the undetermined mass concentrations present a maximum for the continental origin. In these air masses, SO 2-4 is the predominant WSII compound, then NO -3 and NH +4 . In the other sectors, the SO 2-4 and NH +4 concentrations are decreased by a factor comprised from 1.5 to 4.9. For NO -3 , the decrease is much more important, the factor is comprised between 5 and 22. The concentration of Ca2+ is the same for the marine, continental and mediterranean samples whereas it is lower for the marine modified air mass. Because dust source is more important in the Mediterranean region, higher calcium concentration was expected. It has to be kept in mind that our Ca2+ is describing only the water soluble part (about 80 % of the total Ca according to Koçak et al. 2007). It is mainly produced by the heterogeneous reactions between insoluble Ca2+ compound in mineral dust and acidic species in the atmosphere, which are affected by many factors (Clarke and Karani 1992). Consequently, those values are not representative of the total Ca2+. The sea salt has a higher concentration in the marine air mass and is constant in the other air masses.
The PM1, mostly representative of the anthropogenic contribution (Kittelson et al. 2004; Tunved et al. 2006) shows a variable concentration but relatively homogeneous composition (WSII vs undetermined mass) (Fig. 8). In all air masses, the undetermined mass represents the biggest contribution of the mass fraction (Table 6). Within the WSII, despite the variation of the NO -3 /SO 2-4 ratio (between 0.03 and 0.4), SO 2-4 is still the predominant compound and NO -3 is the lowest anthropogenic compounds.
Even if the aerosol sources are expected to differ greatly from one sector to another, we found little difference in the chemical composition of the PM1 fractions in the various air mass types, as observed during seasonal study. At pdD, the PM1 is likely aged, and hence more homogeneous whatever the air mass type.
4.3 PM10-1
Regarding the PM10-1 mass concentration, the maximum is found for the Mediterranean air mass, then the continental air mass and last, as for the PM1, the marine modified and the marine air masses.
The WSII and the undetermined mass concentrations are highest for the mediterranean origin. In detail, SO 2-4 and NH +4 have highest concentrations in continental air masses, as observed for the PM1 whereas the NO -3 has maximum concentration for the mediterranean air masses. The concentration of Ca2+ is the highest in mediterranean samples and then in the continental samples. It is the lowest in marine and marine modified samples. The highest sea salt concentrations are found in the marine and mediterranean air masses. Those concentrations are slightly lower in the modified marine air mass and are minor in the continental air mass.
The PM10-1 concentrations and compositions differ significantly when sampled in one air mass type or the other (Fig. 8). As already mentioned, the presence of NO -3 on the PM10-1 sea salt can be attributed to reaction of NO -3 precursors (e.g., HNO3 and N2O5) with sea salt aerosol Cl- (Harrison and Kitto 1990; Mamane and Gottlieb 1992; Anlauf et al. 2006). In fact, contrarily to what we observed for PM1, PM10-1 NO -3 is the predominant WSII in the particles sampled in the marine - marine modified air masses sampled at pdD. The pdD is situated 320 km from the oceanic coast; hence this result indicates the level of modification of sea salt after it has aged in polluted plumes. Indeed, shorter is the distance between ocean and pdD, smaller is the NO -3 /Na+ ratio (Table 7), in agreement with the observations made by Anlauf et al. (2006). Cavalli et al. (2004) sampled at the coastal western Irish site of Mace Head, and found that NO -3 mass contributes to 1 % on the PM10-1 during spring and autumn, which is more than 10 times lower than our measurements. Regarding sea salt, we find that it can represent up to 23 % of the PM10-1 mass (the highest mass fraction is observed for the marine samples). At Mace Head, it represents 94 % in spring and autumn (Cavalli et al. 2004). In the Mediterranean aerosol, a large fraction of the mass is not WSII, and we suspect that mineral dust is largely contributing to the unaccounted mass, as witnessed by the largest concentrations (but not mass fraction which is not significantly higher compared to other air mass types) of Ca2+ found in these air masses. This is in agreement with the observations of Pey et al. (2009); they observed high concentrations of crustal material and low concentrations of OM + EC and NH4NO3 in the Mediterranean aerosol composition. The SO 2-4 and NH +4 concentrations, which are predominant together with the NO -3 in the PM1, are of minor importance in the PM10-1. In the past, SO 2-4 in this mode has been associated with sea salt aerosols (Harrison and Pio 1983; Anlauf et al. 2006); it can also come from dust particles (Wall et al. 1988; Matsuki et al. 2005). It has been shown by modelling study that the sulphate-deposition rate onto the PM10-1 particles is slow in comparison with that onto the PM1 particles (Song and Carmichael 1999). This would explain why SO 2-4 predominates in the PM1 mode and not in the PM10-1.
We observed that the size-segregated chemical composition of the aerosol is varying with the origin of the air mass as one would expect from the sources associated with each air mass trajectory but still, the variability within an air mass is as important as within a season and we can conclude that both the season and air mass type should be taken into account in order to reduce our uncertainty in attributing an aerosol chemical composition to given environmental conditions.
As a result, we calculate the chemical composition of aerosol as a function of the air mass type and the season (Tables 8, 9, 10 and 11) (refer to Fig. 7 for the number of samples). These tables sum up our previous observations.
Overall, we find that the seasonal variation of major WSII compounds showing a maximum during spring-summer is still observed within a given air mass type. This is also the case for the undetermined mass. For sea salt and Ca2+ concentrations, the seasonal behaviour is not as clear for a given air mass as for the whole data set. This implies that the seasonal variation observed on the whole data set might be driven by the seasonal variation of the air mass types, which shows a higher proportion of mediterranean air masses during autumn and a higher proportion of continental air masses in spring-summer.
5 Conclusion
The measurements conducted at pdD (1,465 m a.s.l.), France, aimed to a better understanding of the influence of the season and air mass origin on the size-segregated chemical composition of the aerosol. Aerosols were sampled using low pressure cascade impactor. The samples were weighed and analysed for water soluble inorganic compounds.
The seasonal variability of the PM1 at pdD showed a maximum in the summer. This can be attributed to 1) a seasonal variability in the mixed layer/free troposphere stratification, 2) a seasonal variation of the aerosol sources. In Venzac et al. (2009), it was shown that the atmospheric vertical dynamics was playing an important role in the seasonal variability of the aerosol number concentration. The absence of seasonal variation of the compounds proportions means that the seasonal variation of sources is not contributing as much to and this confirms that the atmospheric vertical dynamic is a dominant factor influencing the aerosol concentrations at the site.
The PM10-1 exhibited a different seasonal behaviour compared to the PM1, with higher concentrations in the cold season (winter and autumn). During winter, air mass back trajectories are originating from more distant areas, implicating that the average wind speed along the trajectories are higher and larger aerosols such as sea salt can be transported at high altitudes over larger distances.
Aerosols were sampled in different air masses, which could be classified into four different categories according to their origin: marine, marine modified, continental and Mediterranean. The PM10 mass concentration at 50 % relative humidity was close to 2.5 μg m−3 in the marine, 4.3 μg m−3 in the marine modified, 10.3 μg m−3 in the continental and 7.7 μg m−3 in the Mediterranean sectors. We noted that the influence of the air mass origin (on the chemical properties of the aerosol) could be seen after several days especially on the PM10-1. The size distribution of the aerosol varied according to the air mass type. A significant PM10-1 mode was found in marine, modified marine, and Mediterranean air masses, and a dominant PM1 mode in continental air masses. The main WSII constituent of the PM1 was SO 2-4 whatever the air mass origin and for the PM10-1, NO -3 dominated the aerosol composition in all air mass types except for marine air masses in which sea salt dominated the PM10-1.
As a result, the aerosol chemical composition variability at the puy de Dôme is a function of both the season and air mass type and we provide a statistically relevant chemical composition of the aerosol as a function of each of these environmental factors. These chemical characteristics can be used as inputs in future aerosol-cloud process modelling exercises.
References
Andrews, E., Ogren, J.A., Bonasoni, P., Marinoni, A., Cuevas, E., Rodríguez, S., Sun, J.Y., Jaffe, D.A., Fisher, E.V., Baltensperger, U., Weingartner, E., Collaud Coen, M., Sharma, S., Macdonald, A.M., Leaitch, W.R., Lin, N.-H., Laj, P., Arsov, T., Kalapov, I., Jefferson, A., Sheridan, P.: Climatology of aerosol radiative properties in the free troposphere. Atmos Res 102, 365–393 (2011)
Anlauf, K., Li, S.-M., Leaitch, R., Brook, J., Hayden, K., Toom-Sauntry, D., Wiebe, A.: Ionic composition and size characteristics of particles in the Lower Fraser Valley: Pacific 2001 field study. Atmos Environ 40, 2662–2675 (2006)
Asmi, A., Wiedensohler, A., Laj, P., Fjaeraa, A.-M., Sellegri, K., Birmili, W., Weingartner, E., Baltensperger, U., Zdimal, V., Zikova, N., Putaud, J.-P., Marinoni, A., Tunved, P., Hansson, H.-C., Fiebig, M., Kivekäs, N., Lihavainen, H., Asmi, E., Ulevicius, V., Aalto, P.P., Swietlicki, E., Kristensson, A., Mihalopoulos, N., Kalivitis, N., Kalapov, I., Kiss, G., de Leeuw, G., Henzing, B., Harrison, R.M., Beddows, D., O’Dowd, C., Jennings, S.G., Flentje, H., Weinhold, K., Meinhardt, F., Ries, L., Kulmala, M.: Number size distributions and seasonality of submicron particles in Europe 2008–2009. Atmos Chem Phys 11, 5505–5538 (2011)
Baltensperger, U., Gäggeler, H.W., Jost, D.T., Emenegger, M., Nägeli, W.: Continuous background aerosol monitoring with the Epiphaniometer. Atmos Environ 25A, 629–634 (1991)
Bergametti, G., Dutot, A.-L., Buat-Ménard, P., Losno, R., Remoudaki, E.: Seasonal variability of the elemental composition of atmospheric aerosol particles over the northwestern Mediterranean. Tellus 41B, 353–361 (1989)
Birmili, W., Wiedensohler, A.: New particle formation in the continental boundary layer: Meteorological and gas phase parameter influence. Geophys Res Lett 27, 3325–3328 (2000)
Boulon, J., Sellegri, K., Hervo, M., Picard, D., Pichon, J.-M., Fréville, P., Laj, P.: Investigation of nucleation events vertical extent: a long term study at two different altitude sites. Atmos Chem Phys 11, 5625–5639 (2011)
Calvo, A.I., Pont, V., Liousse, C., Dupré, B., Mariscal, A., Zouiten, C., Gardrat, E., Castera, P., Lacaux, C.G., Castro, A., Fraile, R.: Chemical composition of urban aerosols in Toulouse, France during CAPITOUL experiment. Meteorol Atmos Phys 102, 307–323 (2008)
Cavalli, F., Facchini, M.C., Decesari, S., Mircea, M., Emblico, L., Fuzzi, S., Ceburnis, D., Yoon, Y.J., O’Dowd, C.D., Putaud, J.-P., Dell’Acqua, A.: Advances in characterization of size-resolved organic matter in marine aerosol over the North Atlantic. J Geophys Res (2004). doi:10.1029/2004JD005137
Chang, M., Sioutas, C., Cassee, F.R., Fokkens, P.H.B.: Field evaluation of a mobile high-capacity particle size classifier (HCPSC) for separate collection of coarse, fine and ultrafine particles. J Aerosol Sci 32, 139–156 (2001)
Clarke, A.G., Karani, G.N.: Characterisation of the carbonate content of atmospheric aerosols. J Atmos Chem 14, 119–128 (1992)
Cozic, J., Verheggen, B., Weingartner, E., Crosier, J., Bower, K.N., Flynn, M., Coe, H., Henning, S., Steinbacher, M., Henne, S., Collaud Coen, M., Petzold, A., Baltensperger, U.: Chemical composition of free tropospheric aerosol for PM1 and coarse mode at the high alpine site Jungfraujoch. Atmos Chem Phys 8, 407–423 (2008)
Cruz, C.N., Pandis, S.N.: Deliquescence and hygroscopic growth of mixed inorganic_organic atmospheric aerosol. Environ Sci Technol 34, 4313–4316 (2000)
Dougle, P.G., Ten Brink, H.M.: Evaporative losses of ammonium nitrate in nephelometry and impactor measurements. J Aerosol Sci 27(S1), 511–512 (1996)
Draxler, R.R., Rolph, G.D.: HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model access via NOAA ARL READY Website (http://www.arl.noaa.gov/ready/hysplit4.html). NOAA Air Resources Laboratory, Silver Spring, MD (2003)
Gajananda, K., Kuniyal, J.C., Momin, G.A., Rao, P.S.P., Safai, P.D., Tiwari, S., Ali, K.: Trend of atmospheric aerosols over the north western Himalayan region, India. Atmos Environ 39, 4817–4825 (2005)
Hämeri, K., Väkevä, M., Hansson, H.-C., Laaksonen, A.: Hygroscopic growth of the ultrafine ammonium sulphate aerosol measured using an ultrafine tandem differential mobility analyzer. J Geophys Res 105, 22231–22242 (2000)
Hanke, M., Umann, B., Uecker, J., Arnold, F., Bunz, H.: Atmospheric measurements of gas-phase NHO3 and SO2 using chemical ionization mass spectrometry during the MINATROC field campaign 2000 on Monte Cimone. Atmos Chem Phys 3, 417–436 (2003)
Hansson, H.-C., Rood, M.J., Koloutsou-Vakakis, S., Hämeri, K., Orsini, D., Wiedensohler, A.: NaCl aerosol particle hygroscopicity dependence on mixing with organic compounds. J Atmos Chem 31, 321–346 (1998)
Harrison, R.M., Kitto, A.-M.N.: Field intercomparison of filter pack and denuder sampling methods for reactive gaseous and particulate pollutants. Atmos Environ 24A, 2633–2640 (1990)
Harrison, R.M., Pio, C.A.: Size differentiated composition of inorganic atmospheric aerosols of both marine and polluted continental origin. Atmos Environ 17, 1733–1738 (1983)
Heintzenberg, J., Müller, K., Birmili, W., Spindler, G., Wiedensohler, A.: Mass-related aerosol properties over the Leipzig basin. J Geophys Res 103, 13125–13135 (1998)
Jaffe, D., Tamura, S., Harris, J.: Seasonal cycle and composition of background fine particles along the west coast of the US. Atmos Environ 39, 297–306 (2005)
Kasper, A., Puxbaum, H.: Seasonal variation of SO2, HNO3, NH3 and selected aerosol components at Sonnblick (3106 m a.s.l.). Atmos Environ 32, 3925–3939 (1998)
Kerminen, V.-M., Hillamo, R., Teinilä, K., Pakkanen, T., Allegrini, I., Sparani, R.: Ion balances of size-resolved tropospheric aerosol samples: implications for the acidity and atmopsheric processing of aerosols. Atmos Environ 35, 5255–5265 (2001)
Khlystov, A., Kos, G.P.A., ten Brink, H.M., Kruisz, C., Berner, A.: Activation properties of ambient aerosol in The Netherlands. Atmos Environ 30, 3281–3290 (1997)
Khlystov, A.: Cloud forming properties of ambient aerosol in the Netherlands. PhD thesis, University of Wageningen (1998)
Kittelson, D.B., Watts, W.F., Johnson, J.P.: Nanoparticle emissions on Minnesota highways. Atmos Environ 38, 9–19 (2004)
Koçak, M., Mihalopoulos, N., Kubilay, N.: Chemical composition of the fine and coarse mode fraction of aerosols in the northeastern Mediterranean. Atmos Environ 41, 7351–7368 (2007)
Krämer, L., Pöschl, U., Niessner, R.: Microstructural rearrangement of sodium chloride condensation aerosol particles on interaction with water vapour. J Aerosol Sci 31(6), 673–685 (2000)
Krueger, B.J., Grassian, V.H., Cowin, J.P., Laskin, A.: Heterogeneous chemistry of individual mineral dust particles from different dust source regions: the importance of particle mineralogy. Atmos Environ 38, 6253–6261 (2004)
Laj, P., Klausen, J., Bilde, M., Plaß-Duelmer, C., Pappalardo, G., Clerbaux, C., Baltensperger, U., Hjorth, J., Simpson, D., Reimann, S., Coheur, P.-F., Richter, A., De Mazière, M., Rudich, Y., McFiggans, G., Torseth, K., Wiedensohler, A., Morin, S., Schulz, M., Allan, J.D., Attié, J.-L., Barnes, I., Birmili, W., Cammas, J.P., Dommen, J., Dorn, H.-P., Fowler, D., Fuzzi, S., Glasius, M., Granier, C., Hermann, M., Isaksen, I.S.A., Kinne, S., Koren, I., Madonna, F., Maione, M., Massling, A., Moehler, O., Mona, L., Monks, P.S., Müller, D., Müller, T., Orphal, J., Peuch, V.-H., Stratmann, F., Tanré, D., Tyndall, G., Abo Riziq, A., Van Roozendael, M., Villani, P., Wehner, B., Wex, H., Zardini, A.A.: Measuring atmospheric composition change. Atmos Environ 43, 5351–5414 (2009)
Lee, S.C., Ho, K.F., Chan, L.Y., Zielinska, B., Chow, J.C.: Polycyclic aromatic hydrocarbons (PAHs) and carbonyl compounds in urban atmosphere of Hong Kong. Atmos Environ 35, 5949–5960 (2001)
Legrand, M., Puxbaum, H.: Introduction of the CARBOSOL project: Present and Retrospective State of Organic versus Inorganic Aerosol over Europe. J Geophys Res (2007). doi:10.1029/2006JD008271
Lestari, P., Oskouie, A.K., Noll, K.E.: Size distribution and dry deposition of particulate mass, sulfate and nitrate in an urban area. Atmos Environ 37, 2507–2516 (2003)
Mamane, Y., Gottlieb, J.: Nitrate formation on sea-salt and mineral particles – a single particle approach. Atmos Environ 26A, 1763–1769 (1992)
Marenco, F., Bonasoni, P., Calzolari, F., Ceriani, M., Chiari, M., Cristofanelli, P., D’Alessandro, A., Fermo, P., Lucarelli, F., Mazzei, F., Nava, S., Piazzalunga, A., Prati, P., Valli, G., Vecchi, R.: Characterization of atmospheric aerosols at Monte Cimone, Italy, during summer 2004: Source apportionment and transport mechanisms. J Geophys Res (2006). doi:10.1029/2006JD007145
Marinoni, A., Laj, P., Sellegri, K., Mailhot, G.: Cloud chemistry at the Puy de Dôme: variability and relationships with environmental factors. Atmos Chem Phys 4(3), 715–728 (2004)
Martens, C.S., Wesolowski, J.J., Harriss, R.C., Kaifer, R.: Chlorine loss from Puerto Rican and San Francisco bay area marine aerosols. J Geophys Res 78, 8778–8791 (1973)
Martin, G.M., Johnson, D.W., Spice, A.: The measurement and parameterization of effective radius of droplets in warm stratiform clouds. J Atmos Sci 51, 1823–1842 (1994)
Matsuki, A., Iwasaka, Y., Shi, G., Zhang, D., Trochkine, D., Yamada, M., Kim, Y.-S., Chen, B., Nagatani, T., Miyazawa, T., Nagatani, M., and Nakata, H.: Morphological and chemical modification of mineral dust: observational insight into the heterogeneous uptake of acidic gases. Geophys. Res. Lett. 32 (2005). doi:10.1029/2005GL024176
Mészáros, E., Barcza, T., Gelencsér, A., Hlavay, J., Kiss, Gy, Krivácsy, Z., Molnár, A., Polyák, K.: Size distributions of inorganic and organic species in the atmospheric aerosol in Hungary. J Aerosol Sci 28(7), 1163–1175 (1997)
Mikhailov, E., Vlasenko, S., Niessner, R., Oöschl, U.: Interaction of aerosol particles composed of protein and saltswith water vapor: hygroscopic growth and microstructural rearrangement. Atmos Chem Phys 4, 323–350 (2004)
Pakkanen, T.A., Kerminen, V.-M., Hillamo, R.E., Mäkinen, M., Mäkelä, T., Virkulla, A.: Distribution of nitrate over sea-salt and soil derived particles: implications from a field study. J Atmos Chem 24, 189–205 (1996)
Pérez, N., Pey, J., Castillo, S., Viana, M., Alastuey, A., Querol, X.: Interpretation of the variability of levels of regional background aerosols in the Western Mediterranean. Sci Total Environ 407, 527–540 (2008)
Pey, J., Pérez, N., Castillo, S., Viana, M., Moreno, T., Pandolfi, M., López-Sebastián, J.M., Alastuey, A., Querol, X.: Geochemistry of regional background aerosols in the Western Mediterranean. Atmos Res 94, 422–435 (2009)
Pey, J., Pérez, N., Querol, X., Alastuey, A., Cusack, M., Reche, C.: Intense winter atmospheric pollution episodes affecting the Western Mediterranean. Sci Total Environ 408, 1951–1959 (2010)
Pio, C.A., Legrand, M., Oliveira, T., Afonso, J., Santos, C., Caseiro, A., Fialho, P., Barata, F., Puxbaum, H., Sanchez-Ochoa, A., Kasper-Giebl, A., Gelencsér, A., Preunkert, S., Schock, M.: Climatology of aerosol composition (organic versus inorganic) at nonurban sites on a west–east transect across Europe. J Geophys Res (2007). doi:10.1029/2006JD008038
Plate, E., Schulz, M.: Coarse nitrate formation in a coastal area of the North Sea. J Aerosol Sci 28, 333–334 (1997)
Putaud, J.-P., Raes, F., Van Dingenen, R., Brüggemann, E., Facchini, M.C., Decesari, S., Fuzzi, S., Gehrig, R., Hüglin, C., Laj, P., Lorbeer, G., Maenhaut, W., Mihalopoulos, N., Müller, K., Querol, X., Rodriguez, S., Schneider, J., Spindler, G., Ten Brink, H., Tørseth, K., Wiedensohler, A.: A European Aerosol Phenomenology-2: chemical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos Environ 38, 2579–2595 (2004)
Putaud, J.-P., Van Dingenen, R., Alastuey, A., Bauer, H., Birmili, W., Cyrys, J., Flentje, H., Fuzzi, S., Gehrig, R., Hansson, H.C., Harrison, R.M., Herrmann, H., Hitzenberger, R., Hüglin, C., Jones, A.M., Kasper-Giebl, A., Kiss, G., Kousa, A., Kuhlbusch, T.A.J., Löschau, G., Maenhaut, W., Molnar, A., Moreno, T., Pekkanen, J., Perrino, C., Pitz, M., Puxbaum, H., Querol, X., Rodriguez, S., Salma, I., Schwarz, J., Smolik, J., Schneider, J., Spindler, G., Ten Brink, H., Tursic, J., Viana, M., Wiedensohler, A., Raes, F.: A European aerosol phenomenology - 3: Physical and chemical characteristics of particulate matter from 60 rural, urban, and kerbside sites across Europe. Atmos Environ 44, 1308–1320 (2010)
Querol, X., Alastuey, A., Puicercus, J.A., Mantilla, E., Miro, J.V., Lopez-Soler, A., Plana, F., Artiñano, B.: Seasonal evolution of suspended particles around a large coal-fired power station: particulate levels and sources. Atmos Environ 32, 1963–1978 (1998)
Raga, G.B., Jonas, P.R.: On the link between cloud-top radiative properties and sub-cloud aerosol concentrations. Quart J Roy Met Soc 119, 1419–1425 (1993)
Ricard, V., Jaffrezo, J.-L., Kerminen, V.M., Hillamo, R.E., Sillanpaa, S., Ruellan, S., Liousse, C., Cachier, H.: Two years of continuous aerosol measurements in northern Finland. J Geophys Res (2002). doi:10.1029/2001JD000952
Robbins, R.C., Cadle, R.D., Eckhardt, D.L.: The conversion of sodium chloride to hydrogen chloride in the atmosphere. J Meteorol 16, 53–56 (1959)
Rodriguez, S., Querol, X., Alastuey, A., Plana, F.: Sources and processes affecting levels and composition of atmospheric aerosol in the western Mediterranean. J Geophys Res (2002). doi:10.1029/2001JD001488
Rodriguez, S., Querol, X., Alastuey, A., Alarcón, M.M., Mantilla, E., Ruiz, C.R.: Comparative PM10-PM2.5 source contribution study at rural, urban and industrial sites Turing PM episodes in Eastern Spain. Sci Total Environ 328, 95–113 (2004)
Rolph, G.D.: Real-time Environmental Applications and Display sYstem (READY) Website (http://www.arl.noaa.gov/ready/hysplit4.html), NOAA Air Resources Laboratory, Silver Spring, MD (2003)
Röösli, M., Theis, G., Künzli, N., Staehelin, J., Mathys, P., Oglesby, L., Camenzind, M., Braun-Fahrländer, Ch: Temporal and spatial variation of the chemical composition of PM10 at urban and rural sites in the Basel area, Switzerland. Atmos Environ 35, 3701–3713 (2001)
Saliba, N.A., Kouyoumdjian, H., Roumié, M.: Effect of local and long-range transport emissions on the elemental composition of PM10-2.5 and PM2.5 in Beirut. Atmos Environ 41, 6497–6509 (2007)
Salvador, P., Artiñano, B., Pio, C., Afonso, J., Legrand, M., Puxbaum, H., Hammer, S.: Evaluation of aerosol sources at European high altitude backgroung sites with trajectory statistical methods. Atmos Environ 44, 2316–2329 (2010)
Sellegri, K.: Etude du processus d’activation des particules d’aérosol en gouttelettes de nuage: implications en chimie multiphases. PhD thesis, University Joseph Fourrier, Grenoble, France (2002)
Sellegri, K., Laj, P., Peron, F., Dupuy, R., Legrand, M., Preunkert, S., Putaud, J.-P., Cachier, H., Ghermandi, G.: Mass balance of free tropospheric aerosol at the puy de Dôme (France) in winter. J Geophys Res (2003). doi:10.1029/2002JD002747
Song, C.H., Carmichael, G.R.: The aging process of naturally emitted aerosol (sea salt and mineral aerosol) during long range transport. Atmos Environ 33, 2203–2218 (1999)
Sørensen, L.L., Pryor, S.C., de Leeuw, G., Schulz, M.: Flux divergence of nitric acid in the marine atmospheric surface layer. J Geophys Res (2005). doi:10.1029/2004JD005403
Sullivan, R.C., Guazzotti, S.A., Sodeman, D.A., Prather, K.A.: Direct observations of the atmospheric processing of Asian mineral dust. Atmos Chem Phys 7, 1213–1236 (2007)
Ten Brink, H., Otjes, R., Jongejan, P., Kos, G.: Monitoring of the ratio of nitrate to sulphate in size-segregated submicron aerosol in the Netherlands. Atmos Res 92, 270–276 (2009)
Tolocka, M.P., Lake, D.A., Johnston, M.V., Wexler, A.S.: Ultrafine nitrate particle events in Baltimore observed by real-time single particle mass spectrometry. Atmos Environ 38, 3215–3223 (2004)
Tunved, P., Hansson, H.-C., Kerminen, V.-M., Ström, J., Dal Maso, M., Lihavainen, H., Viisanen, Y., Aalto, P.P., Komppula, M., Kulmala, M.: High natural aerosol loading over Boreal Forests. Science 312(5771), 261–263 (2006)
Van Dingenen, R., Raes, F., Putaud, J.-P., Baltensperger, U., Charron, A., Facchini, M.-C., Decesari, S., Fuzzi, S., Gehrig, R., Hansson, H.-C., Harrison, R.M., Hüglin, C., Jones, A.M., Laj, P., Lorbeer, G., Maenhaut, W., Palmgren, F., Querol, X., Rodriguez, S., Schneider, J., Ten Brink, H., Tunved, P., Tørseth, K., Wehner, B., Weingartner, E., Wiedensohler, A., Wåhlin, P.: A European aerosol phenomenology – 1: physical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos Environ 38, 2561–2577 (2004)
Vecchi, R., Bernardoni, V., Fermo, P., Lucarelli, F., Mazzei, F., Nava, S., Prati, P., Piazzalunga, A., Valli, G.: 4-hours resolution data to study PM10 in a “hot spot” area in Europe. Environ Monit Assess 154, 283–300 (2009)
Venzac, H., Sellegri, K., Villani, P., Picard, D., Laj, P.: Seasonal variation of aerosol size distributions in the free troposphere and residual layer at the puy de Dôme station, France. Atmos Chem Phys 9(4), 1465–1478 (2009)
Viana, M., Querol, X., Ballester, F., Llop, S., Esplugues, A., Fernández Patier, R., García Dos Santos, S., Herce, M.D.: Characterising exposure to PM aerosols for an epidemiological study. Atmos Environ 42, 1552–1568 (2008)
Vignati, E., de Leeuw, G., Berkowicz, R.: Modeling coastal aerosol transport and effects of surf-produced aerosols on processes in the marine atmospheric boundary layer. J Geophys Res 106, 20 225–20 238 (2001)
Wall, S.M., John, W., Ondo, J.L.: Measurement of aerosol size distributions for nitrate and major ionic species. Atmos Environ 22, 1649–1656 (1988)
Wang, H.-C., John, W.: Characteristics of the berner impactor for sampling inorganic ions. Aerosol Sci Tech 8, 157–172 (1988)
Weber, R., Orsini, D., Duan, Y., Baumann, K., Kiang, C.S., Chameides, W., Lee, Y.N., Brechtel, F., Klotz, P., Jongejan, P., Ten Brink, H.M., Slanina, J., Boring, C.B., Genfa, Z., Dasgupta, P., Hering, S., Stolzenburg, M., Dutcher, D.D., Edgerton, E., Hartsell, B., Solomon, P., Tanner, R.: Intercomparison of near real time monitors of PM2.5 nitrate and sulphate at the U.S. Environmental Protection Agency Atlanta Supersite. J Geophys Res (2003). doi:10.1029/2001JD001220
Weingartner, E., Burtscher, H., Baltensperger, U.: Hygroscopic properties of carbon and diesel soot particles. Atmos Environ 31(15), 2311–2327 (1997)
Wilson, T.R.S.: Salinity and the major elements of sea-water. In: Riley, J.P., Skirrow, G. (eds.) Chemical oceanography. Academic Press, New York (1975)
Wobrock, W., Flossmann, A.I., Monier, M., Pichon, J.-M., Cortez, L., Fournol, J.-F., Schwarzenböck, A., Mertes, S., Heintzenberg, J., Laj, P., Orsi, G., Ricci, L., Fuzzi, S., Brink, H.T., Jongejan, P., Otjes, R.: The Cloud Ice Mountain Experiment (CIME) 1998: experiment overview and modelling of the microphysical processes during the seeding by isentropic gas expansion. Atmos Res 58(4), 231–265 (2001)
Yan, Z., Bate, S., Chandler, R.E., Isham, V., Wheater, H.: An analysis of daily maximum wind speed in Northwestern Europe using generalized linear models. J Climate 15, 2073–2088 (2002)
Zhang, X.Q., McMurry, P.H.: Theoretical analysis of evaporative losses from impactor and filter surfaces. Atmos Environ 21, 1717–1779 (1987)
Zhang, X.Q., McMurry, P.H.: Evaporation losses of fine particulate nitrates during sampling. Atmos Environ 26, 3305–3312 (1992)
Acknowledgment
The authors gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (http://www.arl.noaa.gov/ready.html) used in this publication. This work has been funded and supported by the Agence gouvernementale De l’Environnement et de la Maîtrise de l’Energie (ADEME).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourcier, L., Sellegri, K., Chausse, P. et al. Seasonal variation of water-soluble inorganic components in aerosol size-segregated at the puy de Dôme station (1,465 m a.s.l.), France. J Atmos Chem 69, 47–66 (2012). https://doi.org/10.1007/s10874-012-9229-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-012-9229-2