Abstract
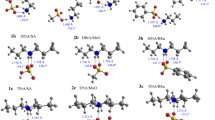
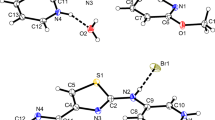
The crystal structures of the proton-transfer compounds of 3,5-dinitrosalicylic acid (DNSA) with a series of aniline-type Lewis bases (aniline, 2-hydroxyaniline, 2-methoxyaniline, 3-methoxyaniline, 4-fluoroaniline, 4-chloroaniline and 2-aminoaniline) have been determined and their hydrogen-bonding systems analysed. All are anhydrous 1:1 salts: [(C6H8N)+(C7H3N2O7)−] (1), [(C6H8NO)+(C7H3N2O7)−] (2), [(C7H10NO)+(C7H3N2O7)−] (3), [(C7H10NO)+(C7H3N2O7)−] (4), [(C6H7FN)+(C7H3N2O7)−] (5), [(C6H7ClN)+(C7H3N2O7)−] (6), and [(C6H9N2)+(C7H3N2O7)−] (7), respectively. Crystals of 1 and 6 are triclinic, space group P-1 while the remainder are monoclinic with space group either P21/n (2, 4, 5 and 7) or P21 (3). Unit cell dimensions and contents are: for 1, a = 7.2027(17), b = 7.5699(17), c = 12.9615(16) Å, α = 84.464(14), β = 86.387(15), γ = 75.580(14)°, Z = 2; for 2, a = 7.407(3), b = 6.987(3), c = 27.653(11) Å, β = 94.906(7)°, Z = 4; for 3, a = 8.2816(18), b = 23.151(6), c = 3.9338(10) Å, β = 95.255(19)°, Z = 2; for 4, a = 11.209(2), b = 8.7858(19), c = 15.171(3) Å, β = 93.717(4)°, Z = 4; for 5, a = 26.377(3), b = 10.1602(12), c = 5.1384(10) Å, β = 91.996(13)°, Z = 4; for 6, a = 11.217(3), b = 14.156(5), c = 4.860(3) Å, α = 99.10(4), β = 96.99(4), γ = 76.35(2)°, Z = 2; for 7, a = 12.830(4), b = 8.145(3), c = 14.302(4) Å, β = 102.631(6)°, Z = 4. In all compounds at least one primary linear intermolecular N+–H⋯O(carboxyl) hydrogen-bonding interaction is present which, together with secondary hydrogen bonding results in the formation of mostly two-dimensional network structures, exceptions being with compounds 4 and 5 (one-dimensional) and compound 6 (three-dimensional). In only two cases (compounds 1 and 4), are weak cation–anion or cation–cation π–π interactions found while weak aromatic C–H⋯O interactions are insignificant. The study shows that all compounds fit the previously formulated classification scheme for primary and secondary interactive modes for proton-transfer compounds of 3,5-dinitrosalicylic acid but there are some unusual variants.
Graphical Abstract
The crystal structure determinations of the anhydrous 1:1 proton-transfer compounds of 3,5-dinitrosalicylic acid with aniline and a set of six monosubstituted anilines (2-hydroxy-, 2-methoxy-, 3-methoxy-, 4-fluoro-, 4-chloro- and 2-aminoaniline) have allowed the hydrogen-bonding systematics to be examined.












Similar content being viewed by others

References
Smith G, Wermuth UD, Bott RC, Healy PC, White JM (2002) Aust J Chem 55:349
Smith G, Wermuth UD, Healy PC, White JM (2002) Aust J Chem 56:707
Smith G, Wermuth UD, Healy PC, White JM (2007) Aust J Chem 60:264
Kumar VSS, Kuduva SS, Desiraju GR (2002) Acta Crystallogr E 58:o865
Smith G, Baldry KE, Byriel KA, Kennard CHL (1997) Aust J Chem 50:727
Smith G, Coyne MG, White JM (2000) Aust J Chem 53:203
Bott RC, Smith G, Wermuth UD, Dwyer NC (2000) Aust J Chem 53:767
Kumar VSS, Nangia A, Katz AK, Carrell HL (2002) Cryst Growth Des 2:313
Smith G, Lynch DE, Byriel KA, Kennard CHL (1995) Aust J Chem 48:1133
Kumar VSS, Kuduva SS, Desiraju GR (1999) J Chem Soc Perkin Trans 2:1069
Smith G, Wermuth UD, White JM (2002) Acta Crystallogr E 58:o1315
Smith G, Wermuth UD, Healy PC, White JM (2006) Aust J Chem 59:320
Smith G, Wermuth UD, Young DJ, Healy PC (2007) Acta Crystallogr E 63:o2517
Smith G, Wermuth UD, Healy PC (2006) Acta Crystallogr E 62:o610
Smith G, Wermuth UD, White JM (2006) Acta Crystallogr C 62:o402
Etter MC, Adsmond D (1990) J Chem Soc Chem Commun, 589
Etter MC, Frankenbach GM (1989) Materials 1:10
Issa YM, Hindawey AM, Issa RM, Nassar AMG (1980) Rev Roum Chim 25:1535
Hindawey AM, Nassar AMG, Issa RM, Issa YM (1980) Ind J Chem A 19:615–619
Issa YM, Hindawey AM, El-Kholy AE, Issa RM (1981) Gazz Chim Ital 111:27
Ng SW, Naumov P, Drew MGB, Wojciechowski G, Brzezinski B (2001) J Mol Struct 595:29
Smith G, Wermuth UD, White JM (2005) Acta Crystallogr C 61:o464
Etter MC, MacDonald JC, Bernstein J (1990) Acta Crystallogr B 46:256
Smith G, Wermuth UD, Bott RC, White JM, Willis AC (2001) Aust J Chem 54:165
Smith G, Wermuth UD, White JM (2001) Aust J Chem 54:171
Song W-D, Guo X–X, Yu L (2007) Acta Crystallogr E 63:o1890
Smith G, Lynch DE, Byriel KA, Kennard CHL (1996) Acta Crystallogr C 52:231
Smith G, Wermuth UD, Healy PC (2002) Acta Crystallogr E 58:o845
Smith G, Wermuth UD, White JM (2005) Acta Crystallogr E 62:o746
Smith G, Bott RC, Wermuth UD (2001) Acta Crystallogr E 57:o640
Mohamed HA, El-Medani SM, Ramadan RM (2005) J Ind Chem Soc 82:799
Subashini A, Samuel E, Muthiah PT, Bocelli G, Cantoni A (2007) Acta Crystallogr E 63:o4049
Sheldrick GM (2008) Acta Crystallogr A 64:112
Sheldrick GM (2008) SHELX97, program for crystal structure determination. University of Göttingen
Molecular Structure Corporation (1999) TeXsan for Windows. Version 1.06. MSC, The Woodlands
Spek AL (2003) J Appl Crystallogr 36:7
Spek AL (2003) PLATON: A crystallographic computing suite
Allen FH, Raithby PR, Shields GP, Taylor R (1998) J Chem Soc Chem Commun, 1034
Sundaralingam M, Jensen LH (1965) Acta Crystallogr 18:1053
Koman M, Martiska L, Valigura D, Glowiak T (2003) Acta Crystallogr E 59:o441
Acknowledgments
The authors acknowledge financial support from the Faculty of Science and Technology, Queensland University of Technology, the School of Biomolecular and Physical Sciences, Griffith University and the School of Chemistry, University of Melbourne.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, G., Wermuth, U.D., Healy, P.C. et al. Structural Systematics of the Anhydrous 1:1 Proton-Transfer Compounds of 3,5-Dinitrosalicylic Acid with Aniline and Monosubstituted Anilines. J Chem Crystallogr 41, 1649–1662 (2011). https://doi.org/10.1007/s10870-011-0153-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0153-0