Abstract
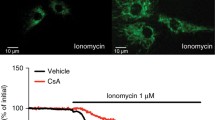
The role of zinc ion in cytotoxicity following ischemic stroke, prolonged status epilepticus, and traumatic brain injury remains controversial, but likely is the result of mitochondrial dysfunction. We describe an excitation ratiometric fluorescence biosensor based on human carbonic anhydrase II variants expressed in the mitochondrial matrix, permitting free zinc levels to be quantitatively imaged therein. We observed an average mitochondrial matrix free zinc concentration of 0.2 pM in the PC12 rat pheochromacytoma cell culture line. Cytoplasmic and mitochondrial free zinc levels were imaged in a cellular oxygen glucose deprivation (OGD) model of ischemia/reperfusion. We observed a significant increase in mitochondrial zinc 1 h following 3 h OGD, at a time point when cytosolic zinc levels were depressed. Following the increase, mitochondrial zinc levels returned to physiological levels, while cytosolic zinc increased gradually over a 24 h time period in viable cells. The increase in intramitochondrial zinc observed during reoxygenation after OGD may contribute to bioenergetic dysfunction and cell death that occurs with both in vitro and in vivo models of reperfusion.
Similar content being viewed by others
References
Aizenman E, Stout AK, Hartnett KA, Dinely KE, McLaughlin B, Reynolds IJ (2000) Induction of neuronal apoptosis by thiol oxidation:putative role of intracellular zinc release. J Neurochem 75(5):1878–1889
Atar D, Backx PH, Appel MM, Gao WD, Marban E (1995) Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J Biol Chem 270(6):2473–2477. doi:10.1074/jbc.270.6.2473
Ayaz M, Turan B (2006) Selenium prevents diabetes-induced alterations in [Zn2+]i and metallothionein level of rat heart via restoration of cell redox cycle. Am J Physiol Heart Circ Physiol 290(3):H1071–H1080. doi:10.1152/ajpheart.00754.2005
Blomgren H, Hagberg H (2006) Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med 40:388–397
Bogaert YE, Rosenthal RE, Fiskum G (1994) Postischemic inhibition of cerebral cortex pyruvate dehydrogenase. Free Radic Biol Med 16(6):811–820
Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E et al (2004) Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 41:351–365
Bozym RA, Thompson RB, Stoddard AK, Fierke CA (2006) Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem Biol 1(2):103–111
Bozym R, Hurst TK, Westerberg N, Stoddard A, Fierke CA, Frederickson CJ et al (2008) Determination of zinc using carbonic anhydrase-based fluorescence biosensors. In: Brand L, Johnson M (eds) Fluorescence Spectroscopy (Vol. 450, Methods in Enzymology). Academic, San Diego, pp 287–309
Bozym RA, Chimienti F, Giblin LJ, Gross GW, Korichneva I, Li Y et al (2010) Free zinc outside a narrow concentration range is toxic to a variety of cells in vitro. Experimental Biology and Medicine 235:741–750. doi:10.1258/ebm.2010.009258
Caporale T, Ciavardelli D, Di Ilio C, Lanuti P, Drago D, Sensi SL (2009) Ratiometric-pericam-mt, a novel tool to evaluate intramitochondrial zinc. Exp Neurol 218(2):228
Danilov CA, Fiskum G (2008) Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. Glia 56(7):801–808. doi:10.1002/glia.20655
Dittmer PJ, Miranda JG, Gorski JA, Palmer AE (2009) Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol Chem 284:16289–16297. doi:10.1074/jbc.M900501200
Dongen EMWMV, Evers TH, Dekkers LM, Meijer EW, Klomp LWJ, Merkx M (2007) Variation of linker length in ratiometric fluorescent sensor proteins allows rational tuning of Zn(II) affinity in the picomolar to femtomolar range. Journal of the American Chemical Society 129:3494–3495. doi:10.1021/ja069105
Fierke CA, Thompson RB (2001) Fluorescence-based biosensing of zinc using carbonic anhydrase. BioMetals 14:205–222
Fiskum G, Murphy AN, Beal MF (1999) Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab 19:351–369
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y et al (2006) Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia, and reperfusion. Exp Neurol 198:285–293. doi:10.1016/j.expneurol.2005.08.030
Galasso SL, Dyck RH (2007) The role of zinc in cerebral ischemia. Molecular Medicine 13(7–8):380–387. doi:10.2119/2007-00044.Galasso
Gazaryan I, Krasnikov BF, Ashby GA, Thorneley RNF, Kristal BS, Brown AM (2002) Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J Biol Chem 277(12):10064–10072
Gazaryan IG, Krasinskaya IP, Kristal BS, Brown AM (2007) Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. J Biol Chem 282:24373–24380. doi:10.107/jbc.M611376200
Haase H, Rink L (2011) Zinc signaling. In: Rink L (ed) Zinc in human health. IOS, Amsterdam
Haase H, Hebel S, Engelhardt G, Rink L (2006) Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem 352(2):222
Haase H, Ober-Blöbaum JL, Engelhardt G, Hebel S, Heit A, Heine H et al (2008) Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol 181(9):6491–6502
Huang C-C, Lesburg CA, Kiefer LL, Fierke CA, Christianson DW (1996) Reversal of the hydrogen bond to zinc ligand histidine-119 dramatically diminishes catalysis and enhances metal equilibration kinetics in carbonic anhydrase II. Biochemistry 35(11):3439–3446
Hunt JA, Ahmed M, Fierke CA (1999) Metal binding specificity in carbonic anhydrase is influenced by conserved hydrophobic amino acids. Biochemistry 38:9054–9060
Hurst TK, Wang D, Thompson RB, Fierke CA (2010) Carbonic anhydrase II-based metal ion sensing: Advances and new perspectives. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics 1804(2):393–403
Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY, Yoon YH (2001) Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42(2):460–465
Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH (2001) Zn2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem 276:47524–47529
Jiang Z-G, Lu XCM, Nelson V, Yang X, Pan W, Chen R-W et al (2006) A multifunctional cytoprotective agent that reduces neurodegeneration after ischemia. Proc Natl Acad Sci U S A 103(5):1581–1586. doi:10.1073/pnas.0510573103
Koh JY, Suh S-W, Gwag BJ, He YY, Hsu CY, Choi DW (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272:1013–1016
Krezel A, Maret W (2006) Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem 11:1049–1062
Lindskog S, Henderson LE, Kannan KK, Liljas A, Nyman PO, Strandberg B (1971) Carbonic anhydrase. In: Boyer PD (ed) The enzymes (Vol. 5, The Enzymes). Academic, New York, pp 587–665
Masanta G, Lim CS, Kim HJ, Han JH, Kim HM, Cho BR (2011) A mitochondrial-targeted two-photon probe for zinc ion. J Am Chem Soc 133(15):5698
McCall KA, Fierke CA (2004) Probing determinants of the metal ion selectivity in carbonic anhydrase using mutagenesis. Biochemistry 43:3979–3986
Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M et al (2010) Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta 1802:92–99
Peck EJ, Ray WJ (1971) Metal complexes of phosphoglucomutase in vivo: alterations induced by insulin. J Biol Chem 246(4):1160–1167
Piantadosi CA, Zhang J (1996) Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27:327–332
Prasad AS, Oberleas D (1968) Zinc in human serum: evidence for an amino acid-bound fraction. J Lab CLin Med 1006
Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE (2011) Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci. doi:10.1037/pnas.1015686108
Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM et al (2004) Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 363(9425):1925
Rudolf E, Červinka M (2010) Zinc pyrithione induces cellular stress signaling and apoptosis in Hep-2 cervical tumor cells: the role of mitochondria and lysosomes. BioMetals 23(2):339–354
Sensi SL, Canoniero LMT, Yu SP, Ying HS, Koh J-Y, Kerchner GA et al (1997) Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci 17(24):9554–9564
Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH (1999) Preferential Zn2+ influx through Ca2+ −permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci 96:2414–2419
Sensi SL, Ton-That D, Weiss JH, Rothe A, Gee KR (2003) A new mitochondrial fluorescent zinc sensor. Cell Calcium 34(3):281–284
Shuttleworth CW, Weiss JH (2011) Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol Sci 32(8):480. doi:j.tips.2011.04.001/j.tips.2011.04.001
Sims NR, Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 1802:80–91
Soane L, Polster BM, Fiskum G (2010) Mitochondrial mechanisms of neural cell death in cerebral ischemia. In: Reed JC, Green D (eds) Apoptosis: physiology and pathology of cell death. Cambridge University Press, Cambridge
Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS et al (2004) Mitochondrial alpha-Ketoglutarate Dehydrogenase Complex Generates Reactive Oxygen Species. J Neurosci 24(36):7779–7788. doi:10.1523/jneurosci.1899-04.2004
Stork CJ, Li YV (2006) Intracellular zinc elevation measured with a "calcium-specific" indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J Neurosci 26(41):10430–10437. doi:10.1523/JNEUROSCI.1588-06.2006
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley-Interscience, New York
Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF et al (2000) Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res 852:268–273
Telford W, Fraker PJ (1995) Preferential induction of apoptosis in mouse CD4+ CD8+ alpha betaTCR10CD3e thymocytes by zinc. J Cell Physiol 164:259–270
Thompson RB (2012) Zinc in stroke: Time for a new approach? In: Li YV, Zhang JH (eds) Metal Ions in Stroke. Springer, Berlin, in the press. doi:10.1007/978-1-4419-9663-3_9
Thompson RB Jr, Whetsell OW, Maliwal BP, Fierke CA, Frederickson CJ (2000) Fluorescence microscopy of stimulated Zn(II) release from organotypic cultures of mammalian hippocampus using a carbonic anhydrase-based biosensor system. J Neurosci Meth 96(1):35–45
Thompson RB, Cramer ML, Bozym R, Fierke CA (2002a) Excitation ratiometric fluorescent biosensor for zinc ion at picomolar levels. J Biomed Opt 7(4):555–560
Thompson RB, Peterson D, Mahoney W, Cramer M, Maliwal BP, Suh SW et al (2002b) Fluorescent zinc indicators for neurobiology. J Neurosci Meth 118:63–75
Tonder N, Johansen FF, Frederickson CJ, Zimmer J, Diemer NH (1990) Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci Lett 109:247–252
Wang D, Hurst TK, Thompson RB, Fierke CA (2011) Genetically encoded ratiometric biosensors to measure extracellular exchangeable zinc in Escherichia coli. J Biomed Opt 16(8):087011. doi:10.1117/1.3613926
Waring P, Egan M, Braithwaite A, Mullbacher N, Siaarda A (1990) Apoptosis induced in macrophages and T-blasts by the mycotoxin sporodesmin and protection by zinc salts. Int J Pharmacol 12:445–457
Wei G, Hough CJ, Li Y, Sarvey JM (2004) Characterization of extracellular accumulation of Zn2+ during ischemia and reperfusion of hippocampus slices in rat. Neuroscience 125:867–877
Weiss JH, Sensi S (2000) Ca2+ −Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci 23(8):365–371
Yin HZ, Sensi SL, Ogoshi F, Weiss JH (2002) Blockade of Ca2+−permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J Neurosci 22(4):1273–1279
Yokoyama M, Koh J, Choi DW (1986) Brief exposure to zinc is toxic to cortical neurons. Neurosci Lett 71:351–355
Zalewski P, Forbes IJ, Giannakis C (1991) Physiological role for zinc in prevention of apoptosis (gene-directed death). Biochem Int 24:1093–1101
Zeng H-H, Bozym RA, Rosenthal RE, Fiskum G, Cotto-Cumba C, Westerberg N, et al. (2005) In situ measurement of free zinc in an ischemia model and cell culture using a ratiometric fluorescence-based biosensor. In T. Vo-Dinh, W. S. Grundfest, D. A. Benaron, & G. E. Cohn (Eds.), SPIE Conference on Advanced Biomedical and CLinical Diagnostic Systems III, San Jose, CA, (Vol. 5692, pp. 51–59): SPIE
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCranor, B.J., Bozym, R.A., Vitolo, M.I. et al. Quantitative imaging of mitochondrial and cytosolic free zinc levels in an in vitro model of ischemia/reperfusion. J Bioenerg Biomembr 44, 253–263 (2012). https://doi.org/10.1007/s10863-012-9427-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9427-2