Abstract
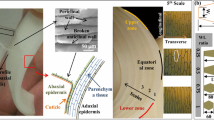
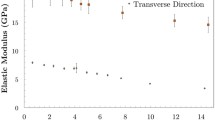
Subcellular mechanical characterization of the cell wall can provide important insights into the cell wall’s functional organization, especially if the characterization is not confounded by extracellular factors and intercellular boundaries. However, due to the technical challenges associated with the microscale mechanical characterization of soft biological materials, subcellular investigations of the plant cell wall under tensile loading have yet to be properly performed. This study reports the mechanical characterization of primary onion epidermal cell wall profiles using a novel cryosection-based sample preparation method and a microelectromechanical system-based tensile testing protocol. At the subcellular scale, the cell wall showed biphasic behavior similar to tissue samples. However, instead of a transition zone between the linear elastic or viscoelastic and linear plastic zones, the subcellular-scale samples showed a plateau-like trend with a sharp drop in the modulus value. The critical ranges of stress (20–40 MPa) and strain (5–12 %) of the plateau zone were identified. A strain energy of 1.3 MJ m−3 was calculated at the midpoint of the critical stress–strain range; this value was in accordance with the previously estimated hydrogen bond energy of the cell wall. Subcellular-scale samples showed very large lateral/axial deformations (0.8 ± 0.13) at fracturing. In addition, investigating the cell wall’s mechanical properties at three different water states showed that water is critical for the flow-like behavior of cell wall matrix polymers. These results at subcellular scale provide new insights into biological materials that possess a structural hierarchy at different length scales; which cannot be obtained from tissue-scale experiments.









Similar content being viewed by others
References
Keegstra K (2010) Plant cell walls. Plant Physiol 154:483–486. doi:10.1104/pp.110.161240
Park YB, Cosgrove DJ (2012) A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol 158:1933–1943
Harris PJ, Stone BA (2008) Chemistry and molecular organization of plant cell walls. Biomass Recalcitrance. Blackwell Publishing Ltd., pp 61–93
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344:1879–1900. doi:10.1016/j.carres.2009.05.021
Burton RA, Gidley MJ, Fincher GB (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6:724–732
Albersheim P, Darvill A, Roberts K (2010) Plant cell walls: from chemistry to biology. Garland Science, Garland.
McCann M, Rose J (2010) Blueprints for building plant cell walls. Plant Physiol 153:365. doi:10.1104/pp.110.900324
Hepworth DG, Bruce DM (2004) Relationships between primary plant cell wall architecture and mechanical properties for onion bulb scale epidermal cells. J Texture Stud 35:586–602
Park YB, Cosgrove DJ (2012) Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of arabidopsis. Plant Physiol 158:465–475. doi:10.1104/pp.111.189779
Cosgrove D, Jarvis M (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3:6
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev cell Biol 6:850–861
Burgert I, Keplinger T (2013) Plant micro- and nanomechanics: experimental techniques for plant cell-wall analysis. J Exp Bot 64:4635–4649. doi:10.1093/jxb/ert255
Liepman AH, Wightman R, Geshi N et al (2010) Arabidopsis—a powerful model system for plant cell wall research. Plant J 61:1107–1121
Somerville C, Bauer S, Brininstool G et al (2004) Toward a systems approach to understanding plant cell walls. Science 306:2206–2211
McCann MC, Carpita NC (2008) Designing the deconstruction of plant cell walls. Curr Opin Plant Biol 11:314–320. doi:10.1016/j.pbi.2008.04.001
Ryden P, Sugimoto-Shirasu K, Smith AC et al (2003) Tensile properties of arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol 132:1033–1040
Suslov D, Verbelen J-P, Vissenberg K (2009) Onion epidermis as a new model to study the control of growth anisotropy in higher plants. J Exp Bot 60:4175–4187
Keckes J, Burgert I, Fruhmann K et al (2003) Cell-wall recovery after irreversible deformation of wood. Nat Mater 2:810–813
Zabler S, Paris O, Burgert I, Fratzl P (2010) Moisture changes in the plant cell wall force cellulose crystallites to deform. J Struct Biol 171:133–141. doi:10.1016/j.jsb.2010.04.013
Zdunek A, Umeda M (2005) Influence of cell size and cell wall volume fraction on failure properties of potato and carrot tissue. J Texture Stud 36:25–43
Pieczywek PM, Zdunek A (2014) Finite element modelling of the mechanical behaviour of onion epidermis with incorporation of nonlinear properties of cell walls and real tissue geometry. J Food Eng 123:50–59. doi:10.1016/j.jfoodeng.2013.09.012
Konstankiewicz K, Pawlak K, Zdunek A (2001) Influence of structural parameters of potato tuber cells on their mechanical properties. Int Agrophys 15:243–246
Waldron KW, Brett CT (2007) The role of polymer cross-linking in intercellular adhesion. In: Roberts JA, Gonzalez-Carranza Z (eds) Plant cell separation and adhesion. Blackwell Publishing Ltd, Ames, pp 183–204
Faisal T, Rey A, Pasini D (2013) A multiscale mechanical model for plant tissue stiffness. Polymers (Basel) 5:730–750. doi:10.3390/polym5020730
Höfte H, Peaucelle A, Braybrook S (2012) Cell wall mechanics and growth control in plants: the role of pectins revisited. Front Plant Sci 3:6
Dick-Pérez M, Zhang Y, Hayes J et al (2011) Structure and Interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50:989–1000. doi:10.1021/bi101795q
Anderson CT, Carroll A, Akhmetova L, Somerville C (2010) Real-time imaging of cellulose reorientation during cell wall expansion in arabidopsis roots. Plant Physiol 152:787–796
Jarvis MC, Briggs SPH, Knox JP (2003) Intercellular adhesion and cell separation in plants. Plant Cell Environ 26:977–989
Gibson LJ (2012) The hierarchical structure and mechanics of plant materials. J R Soc Interface 9:2749–2766. doi:10.1098/rsif.2012.0341
Métraux J-P, Taiz L (1978) Transverse viscoelastic extension in Nitella I. Relationship to growth rate. Plant Physiol 61:135–138
Toole GGA, Gunning PA, Parker MML et al (2001) Fracture mechanics of the cell wall of Chara corallina. Planta 212:606–611. doi:10.1007/s004250000425
Sedighi-Gilani M, Sunderland H, Navi P (2005) Microfibril angle non-uniformities within normal and compression wood tracheids. Wood Sci Technol 39:419–430. doi:10.1007/s00226-005-0022-0
Geitmann A (2006) Experimental approaches used to quantify physical parameters at cellular and subcellular levels. Am J Bot 93:1380–1390. doi:10.3732/ajb.93.10.1380
Peaucelle A (2014) AFM-based Mapping of the elastic properties of cell walls: at tissue, cellular, and subcellular resolutions. e51317. doi:10.3791/51317
Peaucelle A, Braybrook S, Le Guillou L et al (2011) Pectin-induced changes in cell wall mechanics underlie organ initiation in arabidopsis. Curr Biol 21:1720–1726
Kasas S, Longo G, Dietler G (2013) Mechanical properties of biological specimens explored by atomic force microscopy. J Phys D Appl Phys 46:133001
Kasas S, Gmur T, Dietler G (2008) The world of nano-biomechanics. 221–243. doi:10.1016/B978-044452777-6.50014-0
Notbohm J, Poon B, Ravichandran G (2012) Analysis of nanoindentation of soft materials with an atomic force microscope. J Mater Res 27:229–237
Zamil MS, Yi H, Haque A, Virendra MP (2013) Characterizing microscale biological samples under tensile loading: stress-strain behavior of cell wall fragment of onion outer epidermis. Am J Bot 100:1105–1115
Zamil MS, Yi H, Puri VM (2014) Mechanical characterization of outer epidermal middle lamella of onion under tensile loading. Am J Bot 101:778–787. doi:10.3732/ajb.1300416
Kha H, Tuble SC, Kalyanasundaram S, Williamson RE (2010) WallGen, software to construct layered cellulose-hemicellulose networks and predict their small deformation mechanics. Plant Physiol 152:774–786
Yi H, Puri VM (2012) Architecture-based multiscale computational modeling of plant cell wall mechanics to examine the hydrogen-bonding hypothesis of cell wall network structure model. Plant Physiol 160:1281–1292. doi:10.1104/pp.112.201228
Blewett J, Burrows K, Thomas C (2000) A micromanipulation method to measure the mechanical properties of single tomato suspension cells. Biotechnol Lett 22:1877–1883
Saito T, Soga K, Hoson T, Terashima I (2006) The bulk elastic modulus and the reversible properties of cell walls in developing quercus leaves. Plant Cell Physiol 47:715–725
Evered C, Majevadia B, Thompson DS (2007) Cell wall water content has a direct effect on extensibility in growing hypocotyls of sunflower (Helianthus annuus L.). J Exp Bot 58:3361–3371
Moore JP, Farrant JM, Driouich A (2008) A role for pectin-associated arabinans in maintaining the flexibility of the plant cell wall during water deficit stress. Plant Signal Behav 3:102–104
Hansen SL, Ray PM, Karlsson AO et al (2011) Mechanical properties of plant cell walls probed by relaxation spectra. Plant Physiol 155:246–258. doi:10.1104/pp.110.166629
Ulvskov P, Wium H, Bruce D et al (2005) Biophysical consequences of remodeling the neutral side chains of rhamnogalacturonan I in tubers of transgenic potatoes. Planta 220:609–620. doi:10.1007/s00425-004-1373-8
Dick-Perez M, Wang T, Salazar A et al (2012) Multidimensional solid-state NMR studies of the structure and dynamics of pectic polysaccharides in uniformly 13C-labeled Arabidopsis primary cell walls. Magn Reson Chem 50:539–550. doi:10.1002/mrc.3836
Wilson RH, Smith AC, Kačuráková M et al (2000) The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by fourier-transform infrared spectroscopy. Plant Physiol 124:397–406. doi:10.1104/pp.124.1.397
White PB, Wang T, Park YB et al (2014) Water-polysaccharide interactions in the primary cell wall of arabidopsis thaliana from polarization transfer solid-state NMR. J Am Chem Soc 136:10399–10409. doi:10.1021/ja504108h
Zhang T, Mahgsoudy-Louyeh S, Tittmann B, Cosgrove D (2014) Visualization of the nanoscale pattern of recently-deposited cellulose microfibrils and matrix materials in never-dried primary walls of the onion epidermis. Cellulose 21:853–862. doi:10.1007/s10570-013-9996-1
Kafle K, Xi X, Lee CM et al (2014) Cellulose microfibril orientation in onion (Allium cepa L.) epidermis studied by atomic force microscopy (AFM) and vibrational sum frequency generation (SFG) spectroscopy. Cellulose 21:1075–1086. doi:10.1007/s10570-013-0121-2
Engqvist C, Forsberg S, Norgren M et al (2007) Interactions between single latex particles and silica surfaces studied with AFM. Colloids Surf A 302:197–203. doi:10.1016/j.colsurfa.2007.02.032
Tas N, Sonnenberg T, Jansen H, Legtenberg R, Elwenspoek M (1996) Stiction in surface micromachining. J Micromech Microeng 6:385
Evert RF (2006) Esau’s pant anatomy, 3rd edn. Wiley, New York, p 601
Hayles MF, Stokes DJ, Phifer D, Findlay KC (2007) A technique for improved focused ion beam milling of cryo-prepared life science specimens. J Microsc 226:263–269. doi:10.1111/j.1365-2818.2007.01775.x
Vanstreels E, Alamar MC, Verlinden BE et al (2005) Micromechanical behaviour of onion epidermal tissue. Postharvest Biol Technol 37:163–173
Sokolov I, Dokukin ME, Guz NV (2013) Method for quantitative measurements of the elastic modulus of biological cells in AFM indentation experiments. Methods 60:202–213. doi:10.1016/j.ymeth.2013.03.037
Zdunek A, Pieczywek PM (2013) Study on model development of plant tissue using the finite element method. Inside Food Symposium, Leuven, Belgium, pp 9–12
Lee T, Lakes RS (1997) Anisotropic polyurethane foam with Poisson’sratio greater than 1. J Mater Sci 32:2397–2401. doi:10.1023/A:1018557107786
Peel LD (2007) Exploration of high and negative Poisson’s ratio elastomer-matrix laminates. Phys status solidi 244:988–1003. doi:10.1002/pssb.200572717
Greaves GN, Greer AL, Lakes RS, Rouxel T (2011) Poisson’s ratio and modern materials. Nat Mater 10:823–837
Köhler L, Spatz H-C (2002) Micromechanics of plant tissues beyond the linear-elastic range. Planta 215:33–40. doi:10.1007/s00425-001-0718-9
Spatz H, Kohler L, Niklas KJ (1999) Mechanical behaviour of plant tissues: composite materials or structures? J Exp Biol 202:3269–3272
Dintwa E, Jancsók P, Mebatsion HK et al (2011) A finite element model for mechanical deformation of single tomato suspension cells. J Food Eng 103:265–272. doi:10.1016/j.jfoodeng.2010.10.023
Donaldson L (2007) Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci Technol 41:443–460. doi:10.1007/s00226-006-0121-6
Thompson DS (2005) How do cell walls regulate plant growth? J Exp Bot 56:2275–2285
Timoshenko S, Goodier JN (1984) Theory of elasticity, 3rd edn. Singapore, McGraw-Hill, Auckland
Cosgrove D (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Bi 13:171–201
Alamar MC, Vanstreels E, Oey ML et al (2008) Micromechanical behaviour of apple tissue in tensile and compression tests: storage conditions and cultivar effect. J Food Eng 86:324–333
Oey ML, Vanstreels E, De Baerdemaeker J et al (2007) Effect of turgor on micromechanical and structural properties of apple tissue: a quantitative analysis. Postharvest Biol Technol 44:240–247. doi:10.1016/j.postharvbio.2006.12.015
Decraemer WF, Maes MA, Vanhuyse VJ (1980) An elastic stress-strain relation for soft biological tissues based on a structural model. J Biomech 13:463–468. doi:10.1016/0021-9290(80)90338-3
Davies LM, Harris PJ (2003) Atomic force microscopy of microfibrils in primary cell walls. Planta 217:283–289. doi:10.1007/s00425-003-0979-6
Keegstra K, Albersheim P, Darvill A et al (2010) Plant Cell Walls. Plant Physiol 154:483–486. doi:10.1104/pp.110.161240
Hayashi T, Marsden MP, Delmer DP (1987) Pea xyloglucan and cellulose: V. Xyloglucan-cellulose interactions in vitro and in vivo. Plant Physiol 83:384–389
McCann MC, Wells B, Roberts K (1990) Direct visualization of cross-links in the primary plant cell wall. 96:323–334
Ha MA, Apperley DC, Jarvis MC (1997) Molecular rigidity in dry and hydrated onion cell walls. Plant Physiol 115:593–598
Vicré M, Sherwin HW, Driouich A et al (1999) Cell wall characteristics and structure of hydrated and dry leaves of the resurrection plant craterostigma wilmsii, a microscopical study. J Plant Physiol 155:719–726. doi:10.1016/S0176-1617(99)80088-1
Kačuráková M, Smith AC, Gidley MJ, Wilson RH (2002) Molecular interactions in bacterial cellulose composites studied by 1D FT-IR and dynamic 2D FT-IR spectroscopy. Carbohydr Res 337:1145–1153. doi:10.1016/S0008-6215(02)00102-7
Acknowledgements
This study was funded by the Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001090.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zamil, M.S., Yi, H. & Puri, V.M. The mechanical properties of plant cell walls soft material at the subcellular scale: the implications of water and of the intercellular boundaries. J Mater Sci 50, 6608–6623 (2015). https://doi.org/10.1007/s10853-015-9204-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9204-9