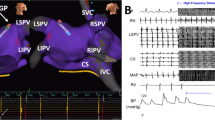
Abstract
Objectives
To study the effects of bilateral vagosympathetic nerve stimulation (VNS) and ganglionated plexi stimulation (GPS) on atrial refractoriness and inducibility of atrial fibrillation (AF).
Methods
Studies were performed in fourteen adult mongrel dogs anesthetized with Na-pentobarbital, 30 mg/kg. VNS was achieved by insertion of wires into the left and right VN trunks. An octapolar catheter was attached to contact the right superior pulmonary vein (RSPV) and other octapolar catheter electrodes were sutured to the right atrial (RA) free wall and appendage (RAA). GPS was performed via a plaque electrode sutured to the fat pad containing the anterior right (AR) GP. VNS and GPS were matched to decrease heart rate by ∼50%. Programmed stimulation delivered from the RSPV or RAA at 2×, 4× and 10× threshold (TH) allowed the determination of atrial refractory period (ARP) and the AF inducibility. The latter was quantitated by the cumulative window of vulnerability (WOV), i.e., the longest minus the shortest coupling interval during which AF was induced at 2×, 4×, 10×, TH combined.
Results
Programmed electrical stimulation at the RSPV showed that the ARP was significantly shorter for both VNS and GPS than baseline (baseline, 113 ± 22 ms; VNS, 94 ± 26 ms; GPS, 85 ± 31 ms) but there was no significant difference in ARP between VNS and GPS. In contrast, the cumulative WOV was significantly wider with GPS (39 ± 36 ms) than either the baseline state (1 ± 1 ms) or with VNS (14 ± 26 ms), p < 0.05. Moreover, pacing from RAA showed a significantly greater cumulative WOV for VNS (33 ± 36 ms) vs both baseline and GPS (1 ± 4 ms and 15 ± 26 ms, respectively, p < 0.05). The heart rate slowing caused by GPS and VNS was not significantly different, 82 ± 11/min vs 82 ± 7/min.
Conclusions
These data indicate a distinct functional separation of autonomic nerve innervation to the atria from the extrinsic and intrinsic nervous systems. AF is more liable to occur due to intrinsic nerve stimulation at the PVs whereas peripheral atrial sites are more readily inducible for AF due to the extrinsic neural input.






Similar content being viewed by others
References
Lewis, T., Drury, A. N., & Bulger, H. A. (1921). Observations upon atrial flutter and fibrillation. VI. Refractory period and rate of propagation in the auricle: Their relation to block in the auricular walls and to flutter etc. Heart, 8, 84–134.
Hoff, H. E., & Geddes, L. A. (1955). Cholinergic factor in atrial fibrillation. Journal of Applied Physiology, 8, 177–192.
Moe, G. K. (1962). On the multiple wavelet hypothesis of atrial fibrillation. Archives Internationales de Pharmacodynamie et de Thérapie, 140, 183–188.
Allessie, M., Lammers, W. J. E. P., Boake, F. I. M., & Hollen, J. (1985). Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In D. P. Zipes, & Jalife (Eds.), Cardiac electrophysiology and arrhythmias (pp. 265–275). New York: Grune and Stratton.
Chiou, C. W., Eble, J. N., & Zipes, D. P. (1997). Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. Circulation, 95, 2573–2584.
Randall, W. C. (1977). Neural regulation of the heart. New York: Oxford University Press.
Randall, W. C. (1984). Nervous control of cardiovascular function. New York: Oxford University Press.
Ardell, J. L. (1994). Structure and function of the mammalian intrinsic cardiac neurons. In J. A. Armour, & J. L. Ardell (Eds.), Neurocardiology. New York: Oxford University Press Chap 5.
Scherlag, B. J., Yamanashi, W. S., Patel, U., Lazzara, R., & Jackman, W. M. (2005). Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. Journal of the American College of Cardiology, 45, 1878–1886.
Po, S. S., Scherlag, B. J., Yamanashi, W. S., et al. (2006). Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein–atrial junctions. Heart Rhythm, 3, 201–208.
Platt, M., Mandapati, R., Scherlag, B. J., et al. (2004). Limiting the number and extent of radiofrequency applications to terminate atrial fibrillation and subsequently prevent its inducibility [abstract]. Heart Rhythm, 1, S11.
Pappone, C., Santinelli, V., Manguso, F., et al. (2004). Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation, 109, 327–334.
Scherlag, B. J., Nakagawa, H., Jackman, W. M., et al. (2005). Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 13, 37–42.
Lemery, R., Bimie, D., Tang, A. S., Green, M., & Gollob, M. (2006). Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm, 3, 387–396.
Lazzara, R., Scherlag, B. J., Robinson, M. J., et al. (1973). Selective in situ parasympathetic control of the canine sinoatrial and atrioventricular nodes. Circulation Research, 32, 393–401.
Priola, D. (1972). Positive dromotropic effects of the vagus nerves on the canine atrioventricular node. Cardiovascular Research, 6, 155–162.
Patterson, E., Po, S., Scherlag, B. J., & Lazzara, R. (2005). Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm, 2, 624–631.
Quan, K. J., Lee, J. H., Geha, A. S., et al. (1999). Characterization of sinoatrial parasympathetic innervation in humans. Journal of Cardiovascular Electrophysiology, 10, 1060–1065.
Quan, K. J., Lee, J. H., Van Hare, G. F., et al. (2002). Identification and characterization of atrioventricular parasympathetic innervation in humans. Journal of Cardiovascular Electrophysiology, 13, 735–739.
Zhou, J., Scherlag, B. J., Edwards, J., Jackman, W. M., Lazzara, R., & Po, S. S. (2007). Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. Journal of Cardiovascular Electrophysiology, 18, 83–90.
Acknowledgement
We thank Mrs. Andrea Moseley for her technical and secretarial assistance and Joseph Klimkoski and Tushar Sharma for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported, in part by grant #0650077Z from the American Heart Association (SSP), grant #K23HL069972 from the National Heart, Lung and Blood Institute (SSP) and from the Helen and Wil Webster Research Fund of the Oklahoma University Research Foundation.
Rights and permissions
About this article
Cite this article
Zhang, Y., Scherlag, B.J., Lu, Z. et al. Comparison of atrial fibrillation inducibility by electrical stimulation of either the extrinsic or the intrinsic autonomic nervous systems. J Interv Card Electrophysiol 24, 5–10 (2009). https://doi.org/10.1007/s10840-008-9297-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-008-9297-z